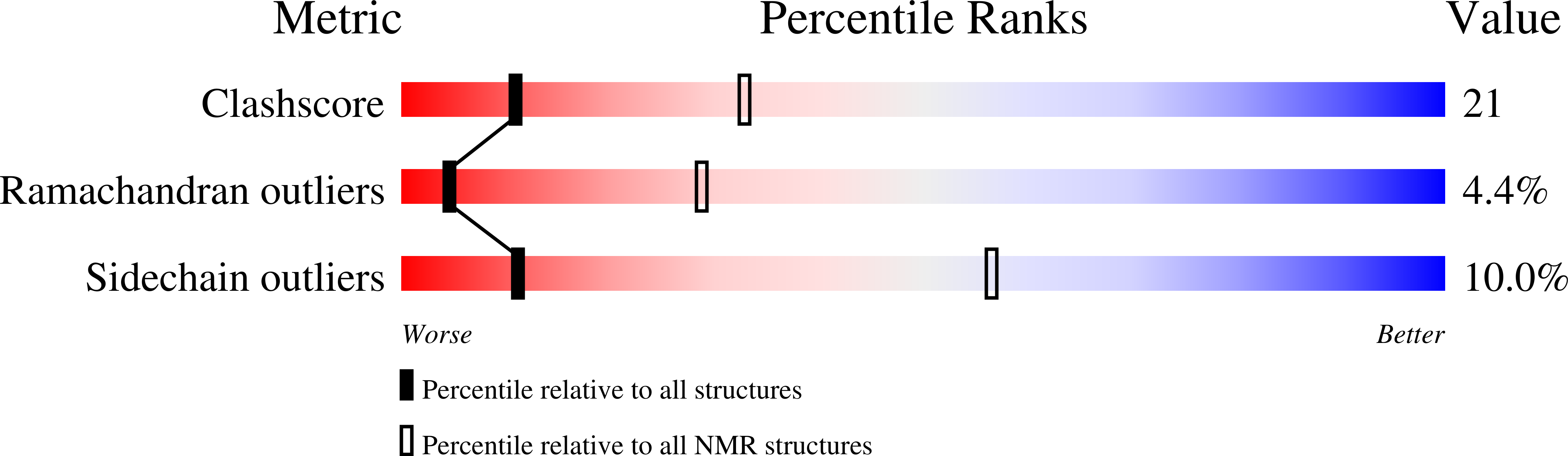
Deposition Date
2005-03-16
Release Date
2005-08-09
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1Z4H
Keywords:
Title:
The response regulator TorI belongs to a new family of atypical excisionase
Biological Source:
Source Organism:
Escherichia coli (Taxon ID: 562)
Host Organism:
Method Details:
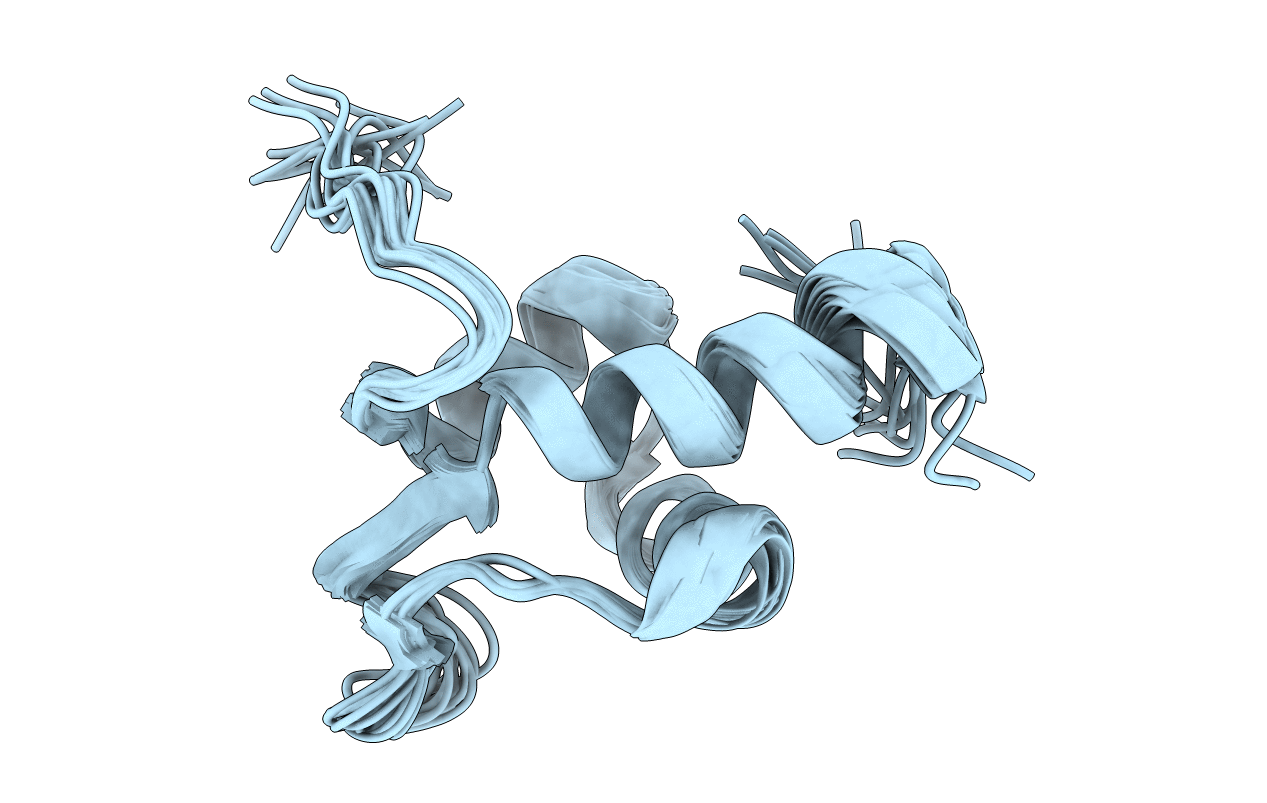
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
17
Selection Criteria:
structures with the least restraint violations