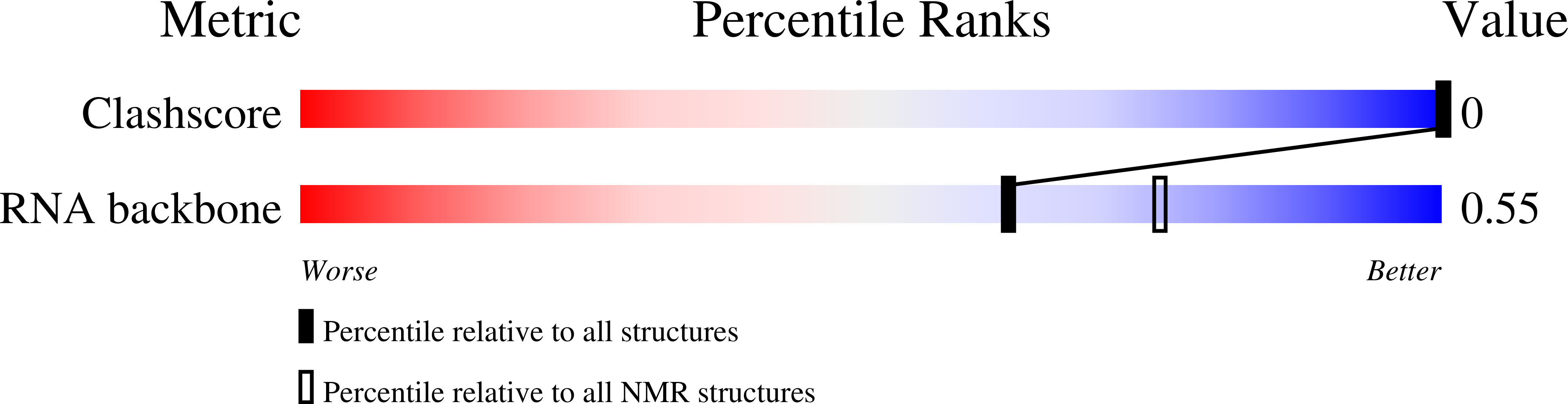
Deposition Date
2005-03-10
Release Date
2005-04-26
Last Version Date
2024-05-29
Entry Detail
PDB ID:
1Z30
Keywords:
Title:
NMR structure of the apical part of stemloop D from cloverleaf 1 of bovine enterovirus 1 RNA
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
15
Selection Criteria:
structures with the lowest energy, target function