Deposition Date
2005-02-22
Release Date
2005-04-26
Last Version Date
2024-02-14
Entry Detail
PDB ID:
1YXN
Keywords:
Title:
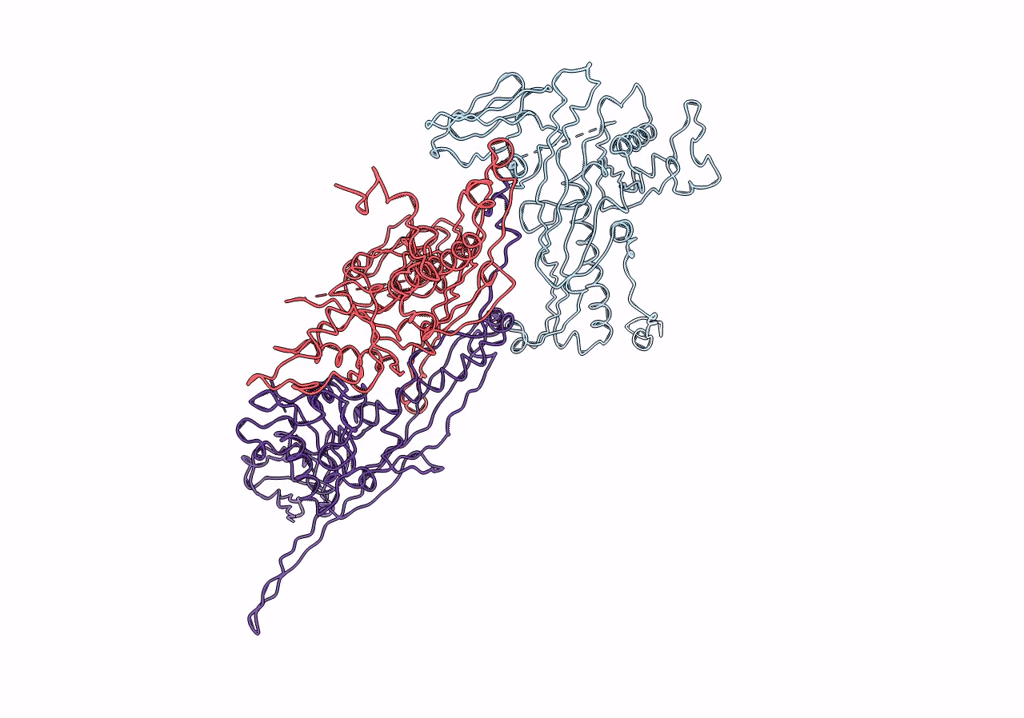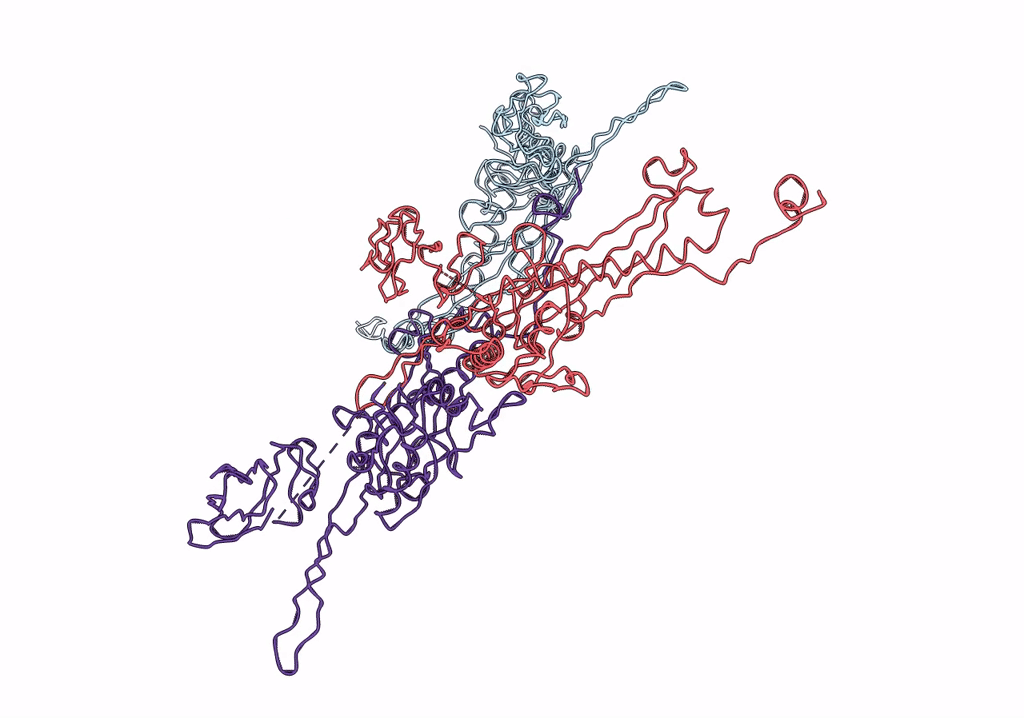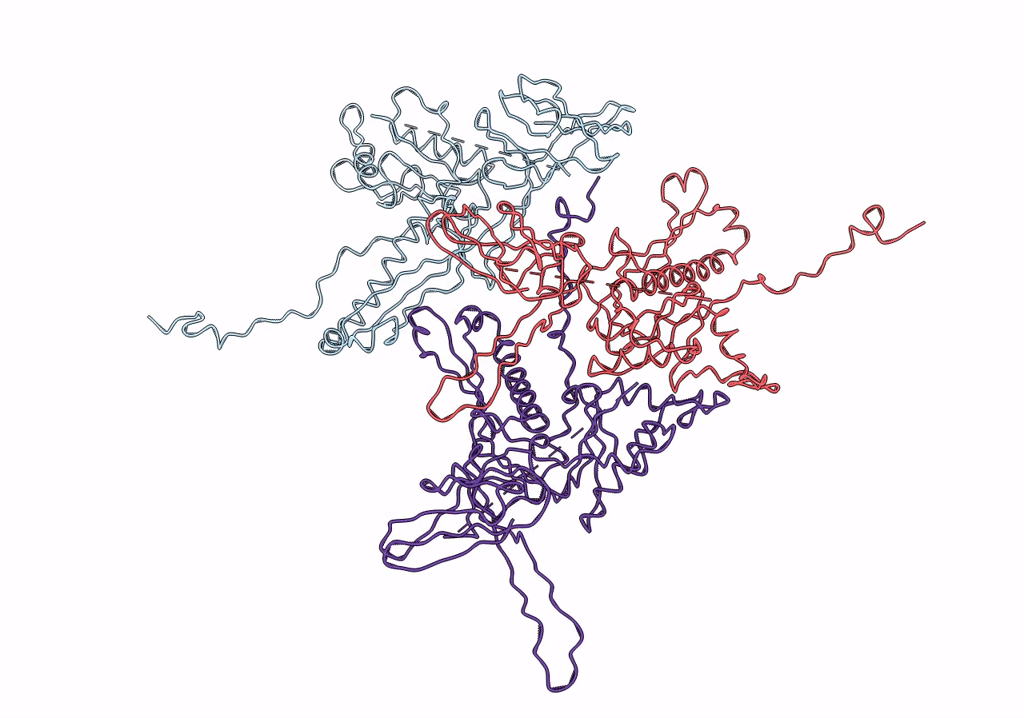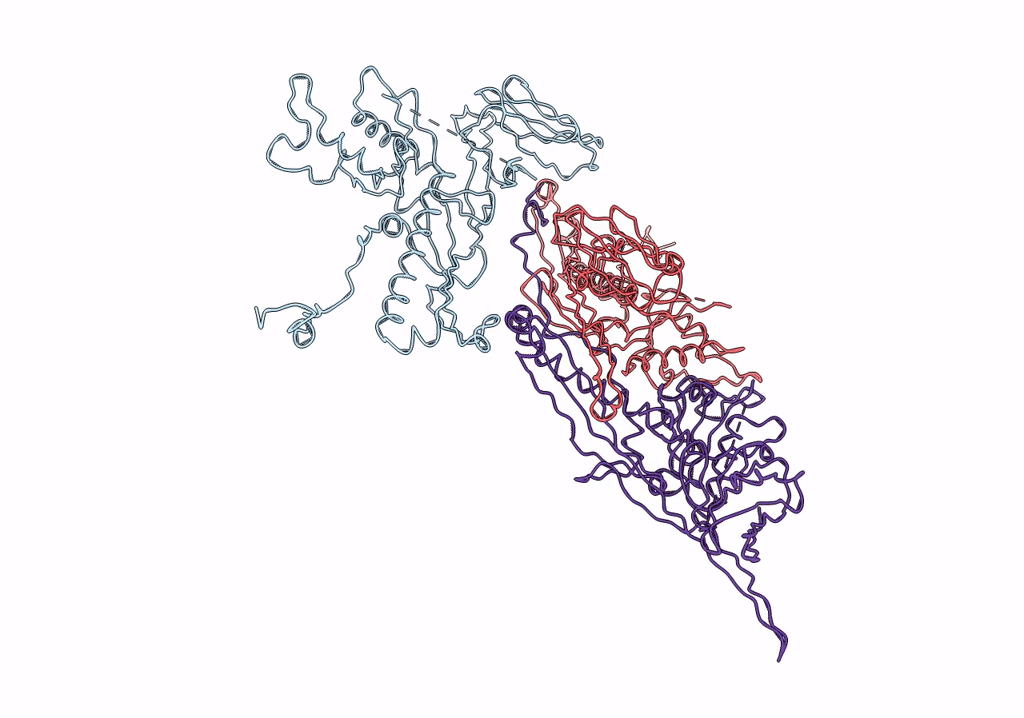
Pseudo-atomic model of a fiberless isometric variant of bacteriophage phi29
Biological Source:
Source Organism:
Bacillus phage phi29 (Taxon ID: 10756)
Host Organism:
Method Details:
Experimental Method:
Resolution:
7.90 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE