Deposition Date
2005-02-11
Release Date
2005-04-12
Last Version Date
2023-09-20
Entry Detail
PDB ID:
1YTV
Keywords:
Title:
Maltose-binding protein fusion to a C-terminal fragment of the V1a vasopressin receptor
Biological Source:
Source Organism:
Escherichia coli (Taxon ID: 562)
Homo sapiens (Taxon ID: 9606)
Homo sapiens (Taxon ID: 9606)
Host Organism:
Method Details:
Experimental Method:
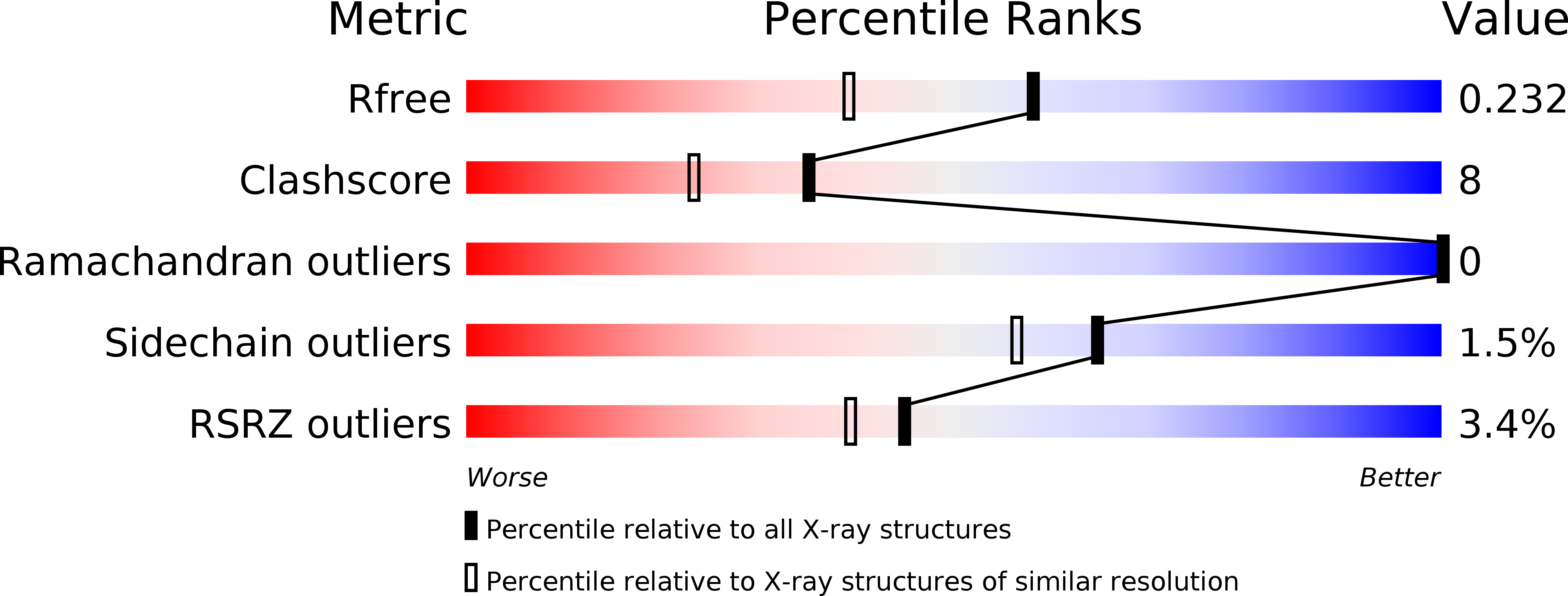
Resolution:
1.80 Å
R-Value Free:
0.22
R-Value Work:
0.19
Space Group:
P 1 21 1