Deposition Date
2005-02-09
Release Date
2005-06-14
Last Version Date
2023-08-23
Entry Detail
PDB ID:
1YSZ
Keywords:
Title:
Crystal Structure of the Unliganded Form of GRP94, the ER Hsp90: Basis for Nucleotide-Induced Conformational Change, GRP94N(DELTA)41 APO CRYSTAL SOAKED WITH NECA
Biological Source:
Source Organism(s):
Canis lupus familiaris (Taxon ID: 9615)
Expression System(s):
Method Details:
Experimental Method:
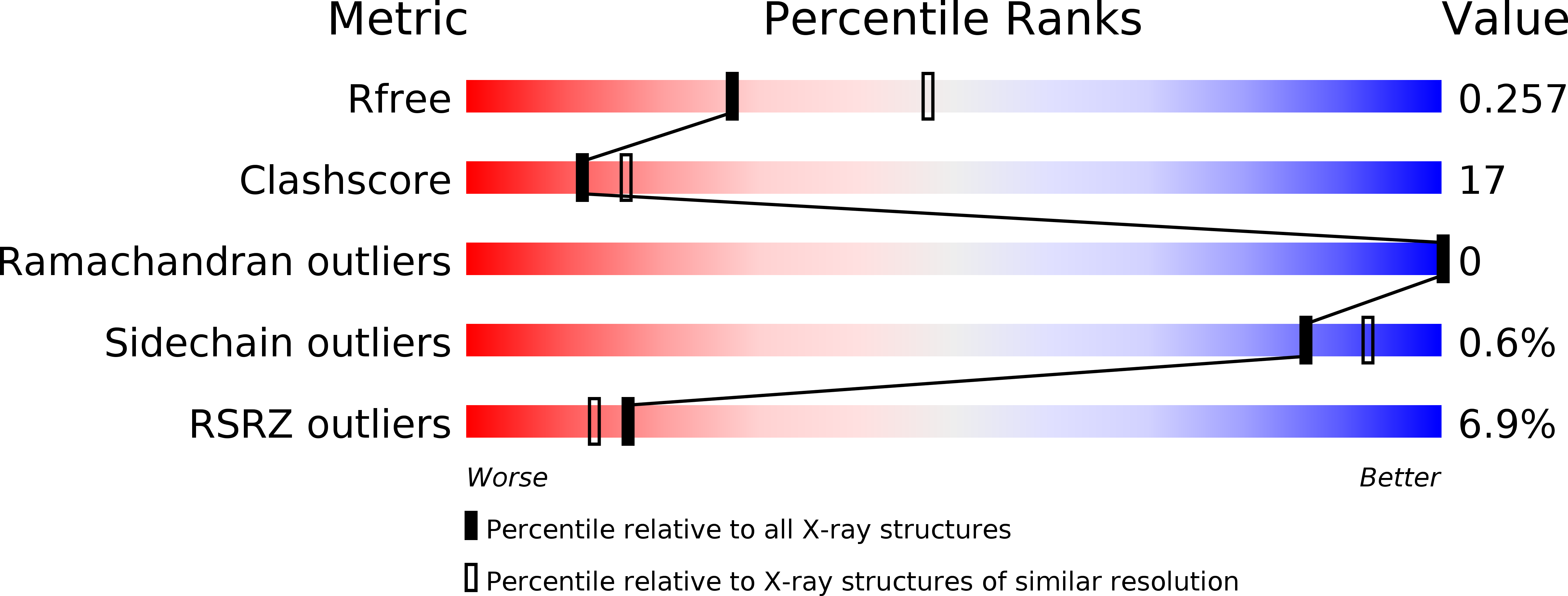
Resolution:
2.65 Å
R-Value Free:
0.26
R-Value Work:
0.21
R-Value Observed:
0.21
Space Group:
P 21 21 2