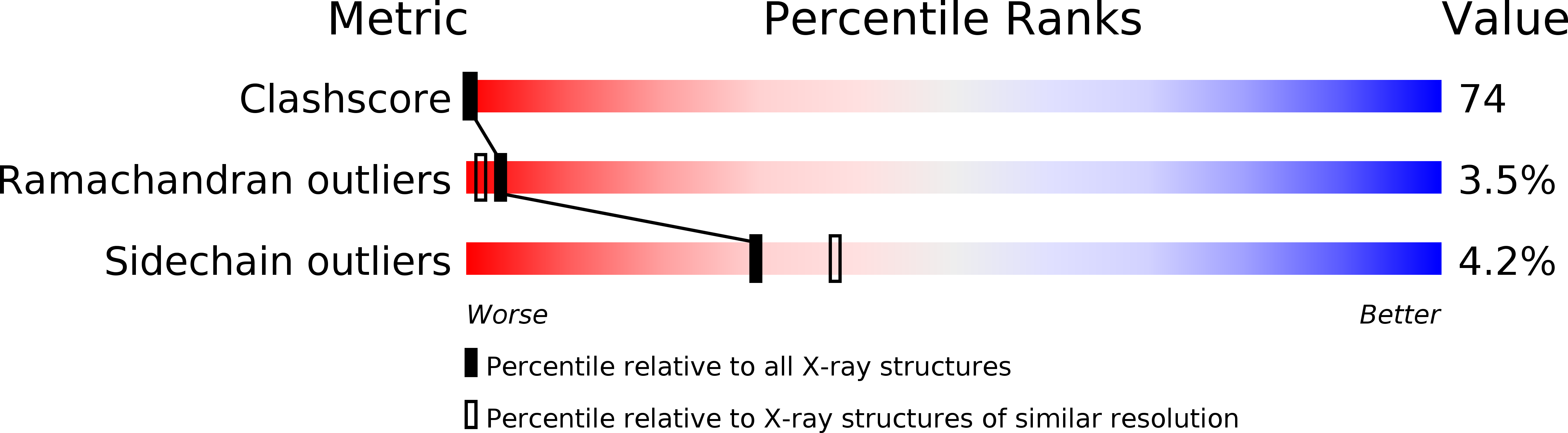
Deposition Date
2005-01-21
Release Date
2005-05-10
Last Version Date
2023-08-23
Entry Detail
PDB ID:
1YMP
Keywords:
Title:
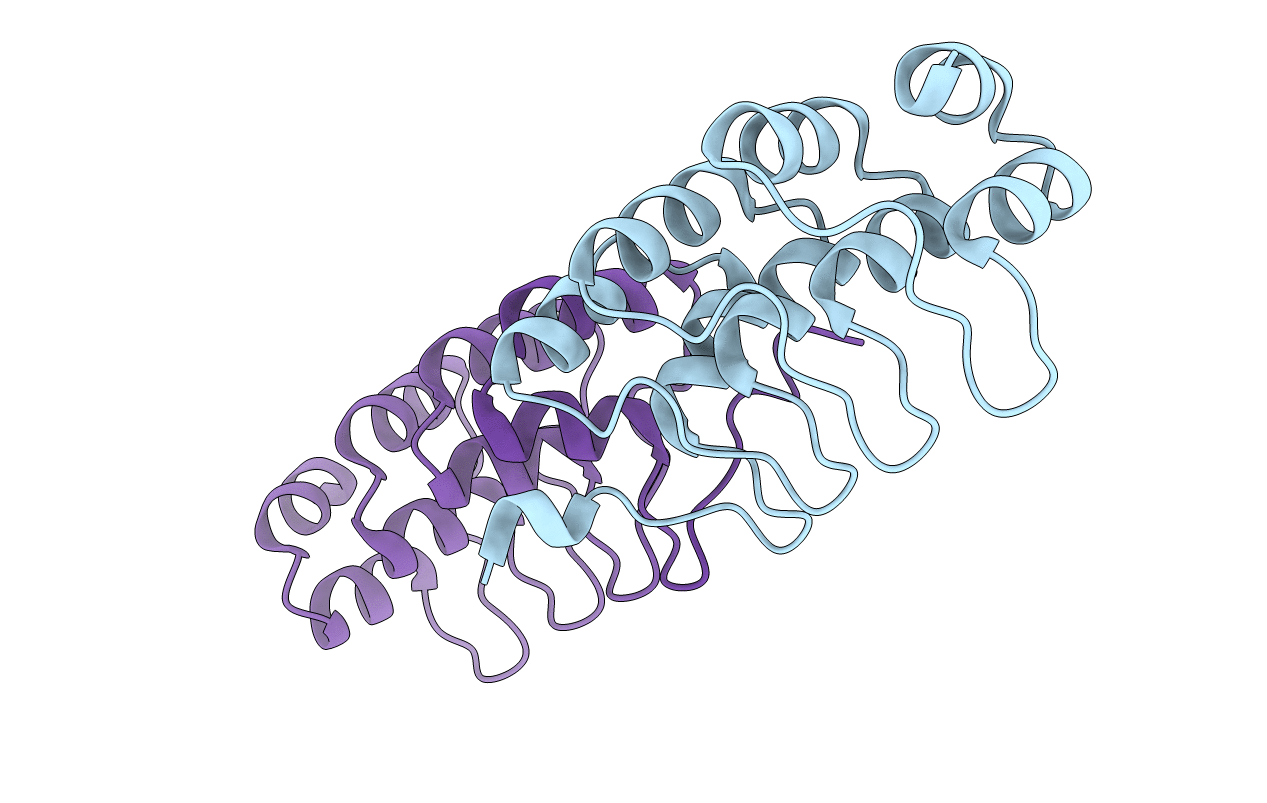
The Crystal Structure of a Partial Mouse Notch-1 Ankyrin Domain: Repeats 4 Through 7 Preserve an Ankyrin Fold
Biological Source:
Source Organism:
Mus musculus (Taxon ID: 10090)
Host Organism:
Method Details:
Experimental Method:
Resolution:
2.20 Å
R-Value Free:
0.30
R-Value Work:
0.20
Space Group:
P 31