Deposition Date
2005-01-14
Release Date
2006-01-24
Last Version Date
2023-08-23
Entry Detail
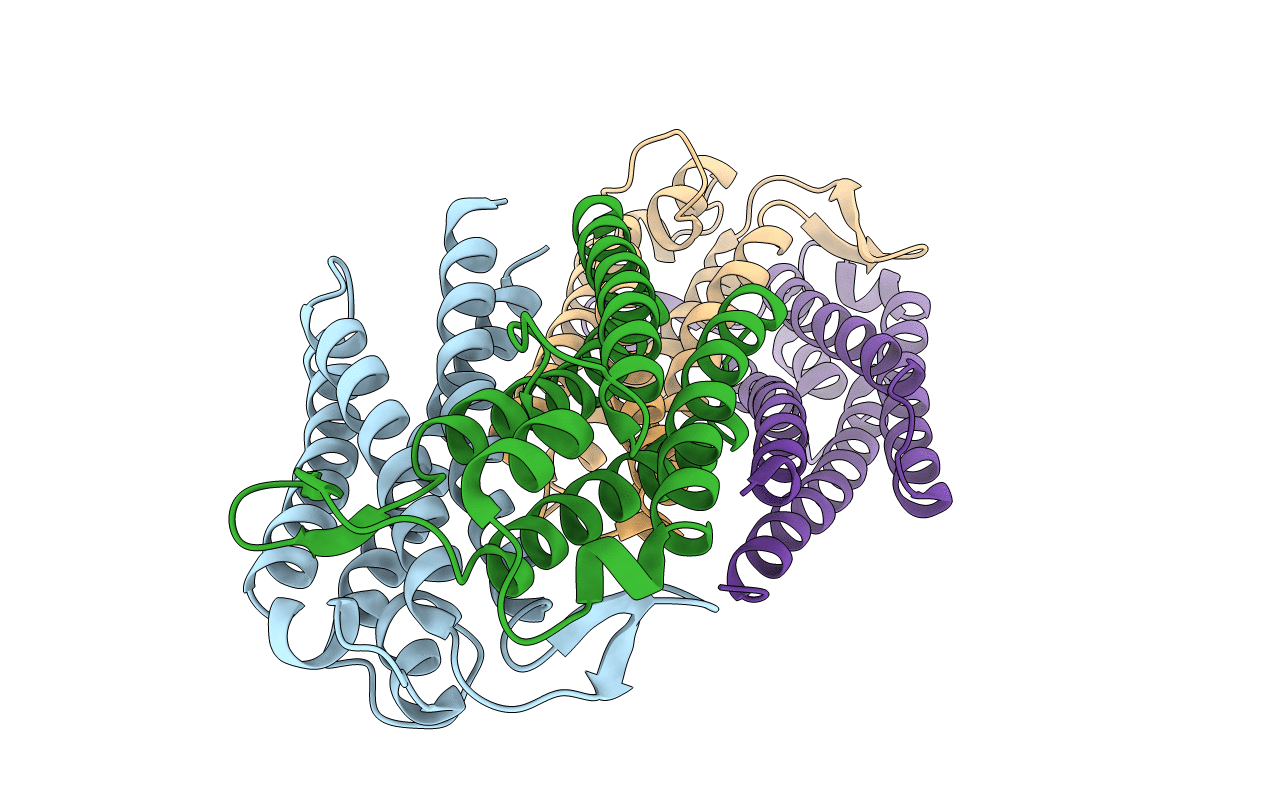
PDB ID:
1YJG
Keywords:
Title:
Variable Small Protein 1 of Borrelia turicatae (VspA or Vsp1)
Biological Source:
Source Organism:
Borrelia turicatae (Taxon ID: 142)
Host Organism:
Method Details:
Experimental Method:
Resolution:
2.22 Å
R-Value Free:
0.26
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
C 1 2 1


