Deposition Date
1993-01-06
Release Date
1993-10-31
Last Version Date
2024-02-14
Entry Detail
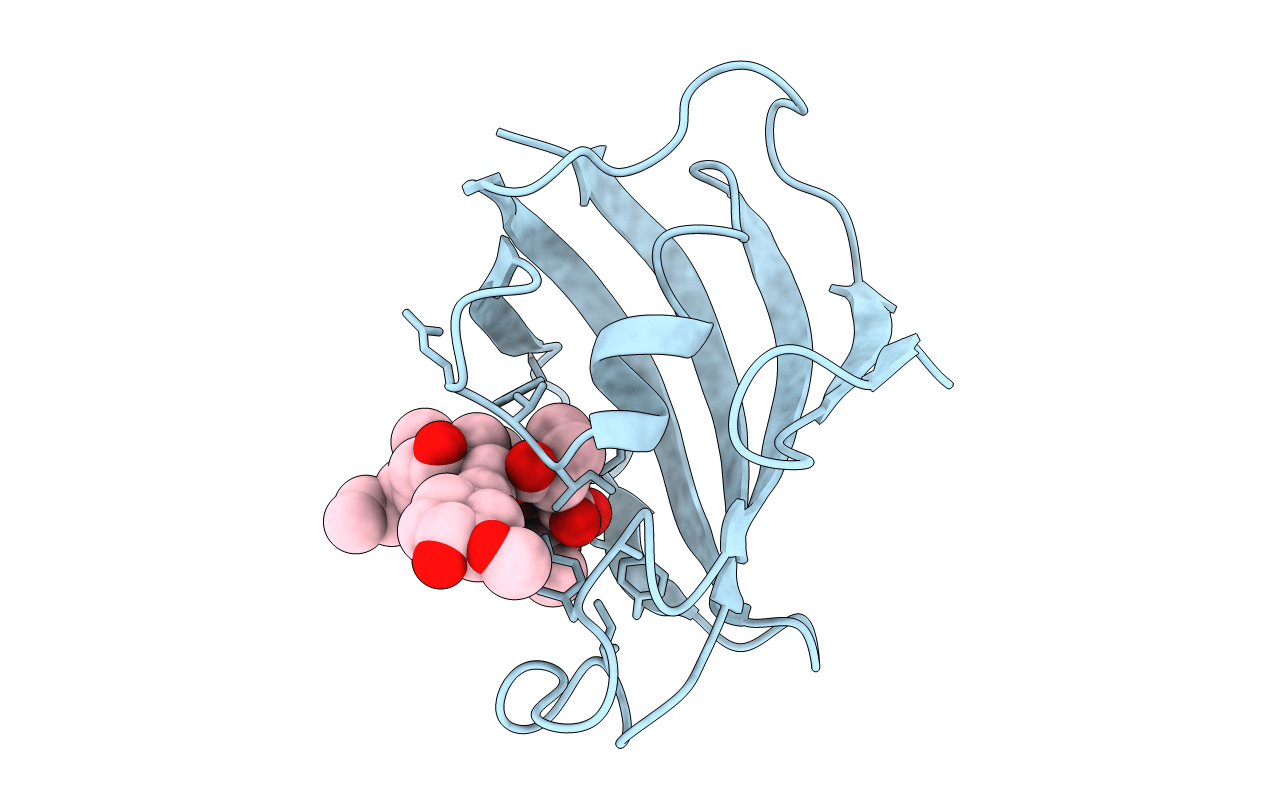
PDB ID:
1YAT
Keywords:
Title:
IMPROVED CALCINEURIN INHIBITION BY YEAST FKBP12-DRUG COMPLEXES. CRYSTALLOGRAPHIC AND FUNCTIONAL ANALYSIS
Biological Source:
Source Organism(s):
Saccharomyces cerevisiae (Taxon ID: 4932)
Method Details:


