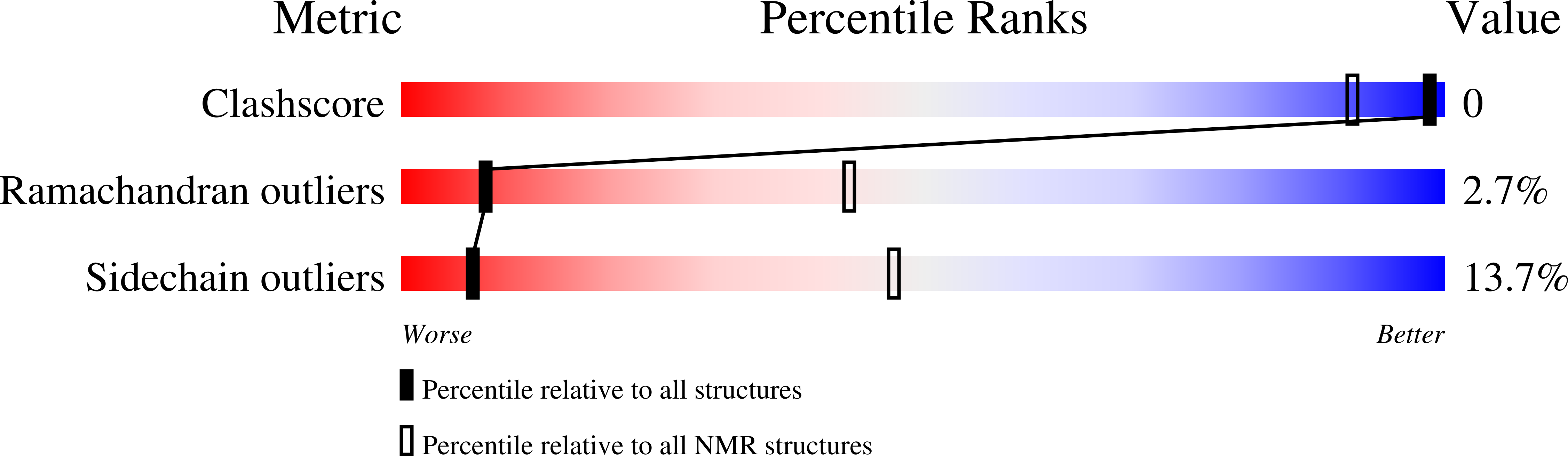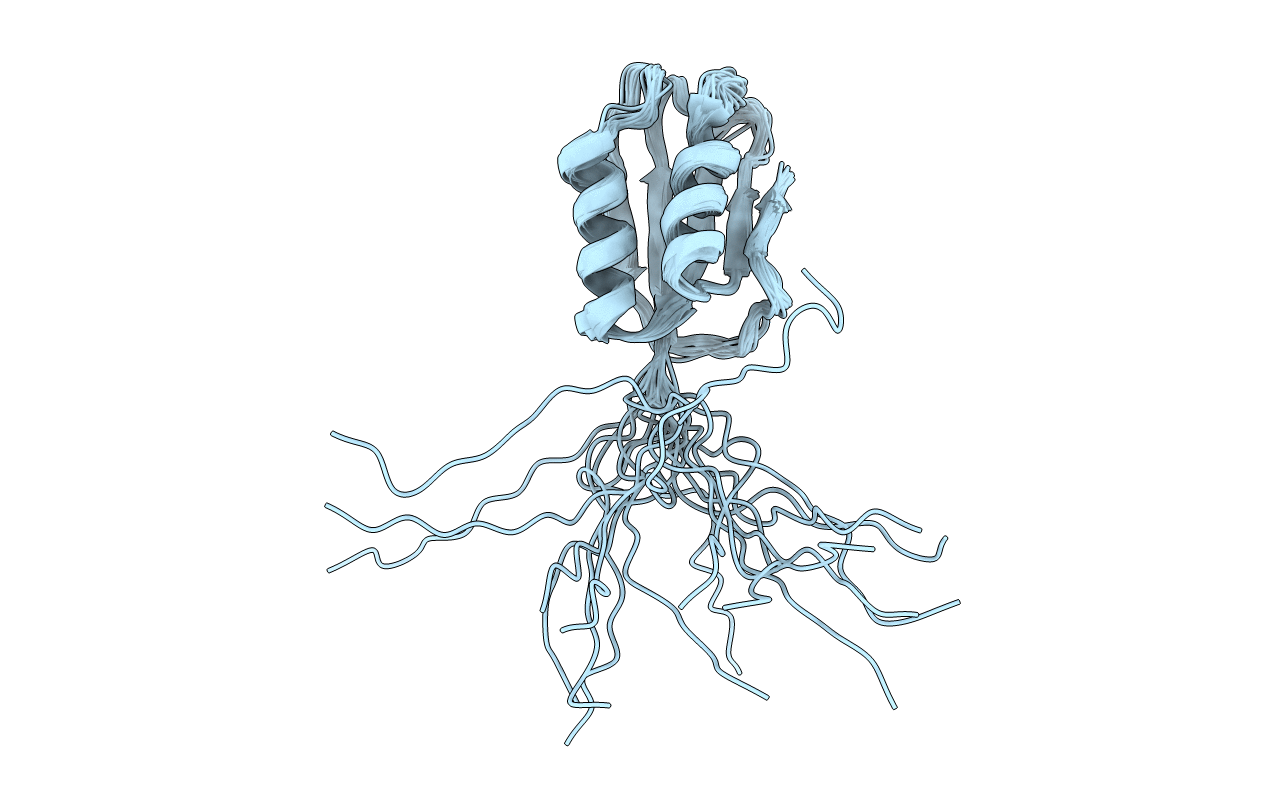
Deposition Date
2004-12-16
Release Date
2005-11-29
Last Version Date
2024-05-29
Entry Detail
PDB ID:
1Y9O
Keywords:
Title:
1H NMR Structure of Acylphosphatase from the hyperthermophile Sulfolobus Solfataricus
Biological Source:
Source Organism:
Sulfolobus solfataricus (Taxon ID: 2287)
Host Organism:
Method Details:
Experimental Method:
Conformers Calculated:
20
Conformers Submitted:
20
Selection Criteria:
target function