Deposition Date
2004-12-15
Release Date
2005-03-22
Last Version Date
2024-04-24
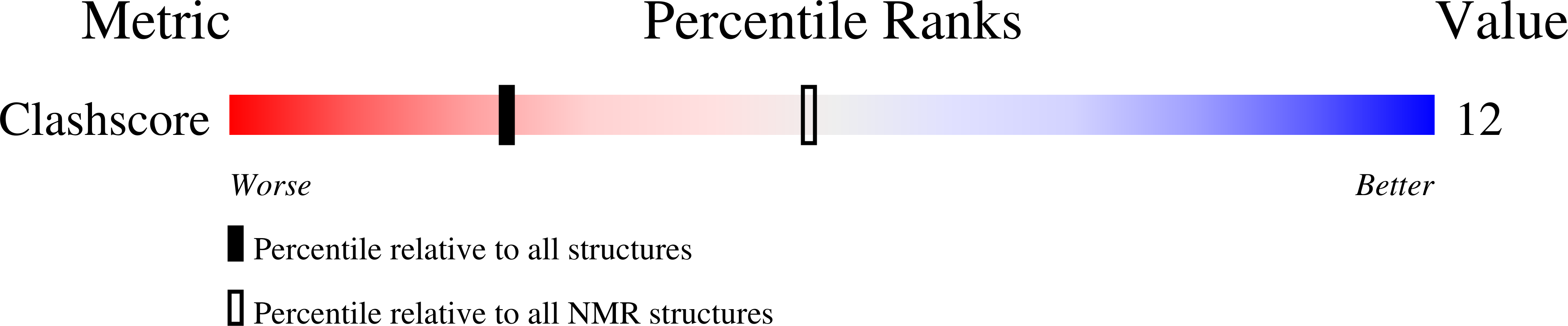
Entry Detail
PDB ID:
1Y9H
Keywords:
Title:
Methylation of cytosine at C5 in a CpG sequence context causes a conformational switch of a benzo[a]pyrene diol epoxide-N2-guanine adduct in DNA from a minor groove alignment to intercalation with base displacement
Method Details:
Experimental Method:
Conformers Calculated:
9
Conformers Submitted:
9
Selection Criteria:
all calculated structures submitted,back calculated data agree with experimental NOESY spectrum,structures with acceptable covalent geometry,structures with favorable non-bond energy,structures with the least restraint violations,structures with the lowest energy,target function