Deposition Date
2004-12-08
Release Date
2004-12-21
Last Version Date
2024-11-13
Entry Detail
PDB ID:
1Y7I
Keywords:
Title:
Structural and biochemical studies identify tobacco SABP2 as a methylsalicylate esterase and further implicate it in plant innate immunity, Northeast Structural Genomics Target AR2241
Biological Source:
Source Organism(s):
Nicotiana tabacum (Taxon ID: 4097)
Expression System(s):
Method Details:
Experimental Method:
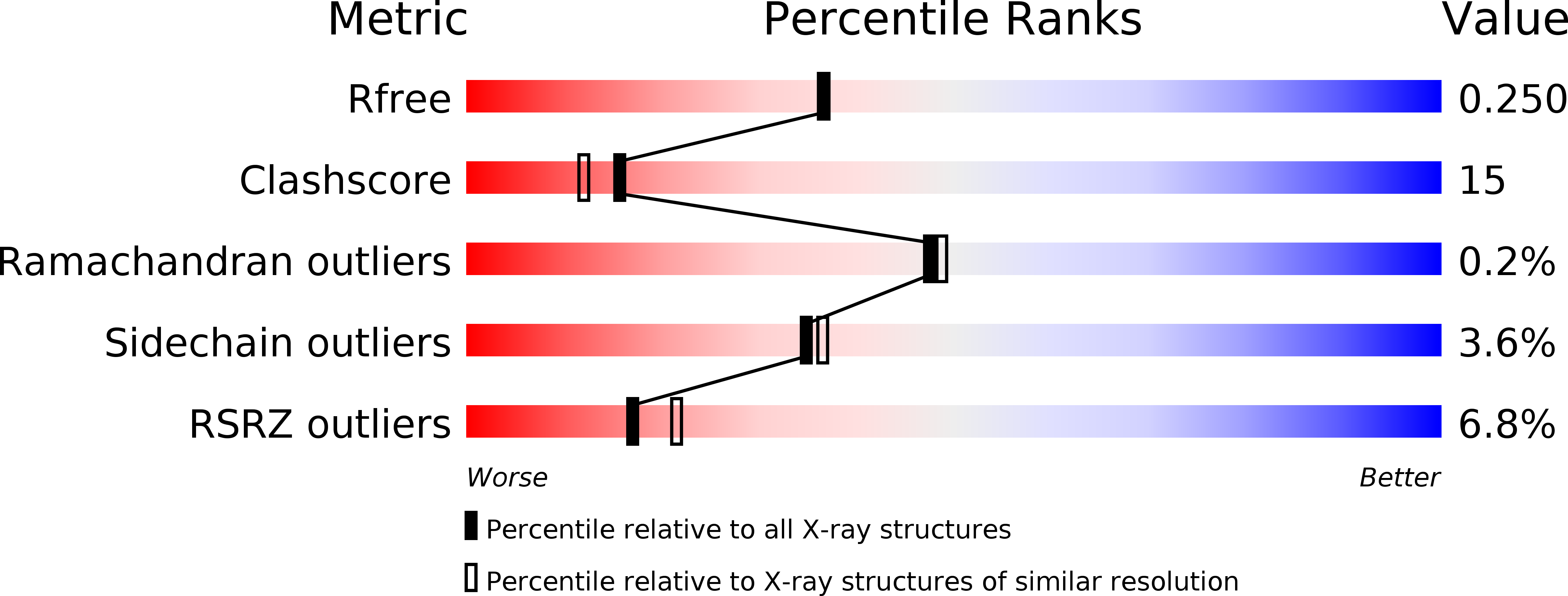
Resolution:
2.10 Å
R-Value Free:
0.24
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
C 1 2 1