Deposition Date
2004-11-01
Release Date
2005-10-11
Last Version Date
2024-03-13
Entry Detail
PDB ID:
1XWJ
Keywords:
Title:
Vinculin head (1-258) in complex with the talin vinculin binding site 3 (1945-1969)
Biological Source:
Source Organism:
Gallus gallus (Taxon ID: 9031)
Host Organism:
Method Details:
Experimental Method:
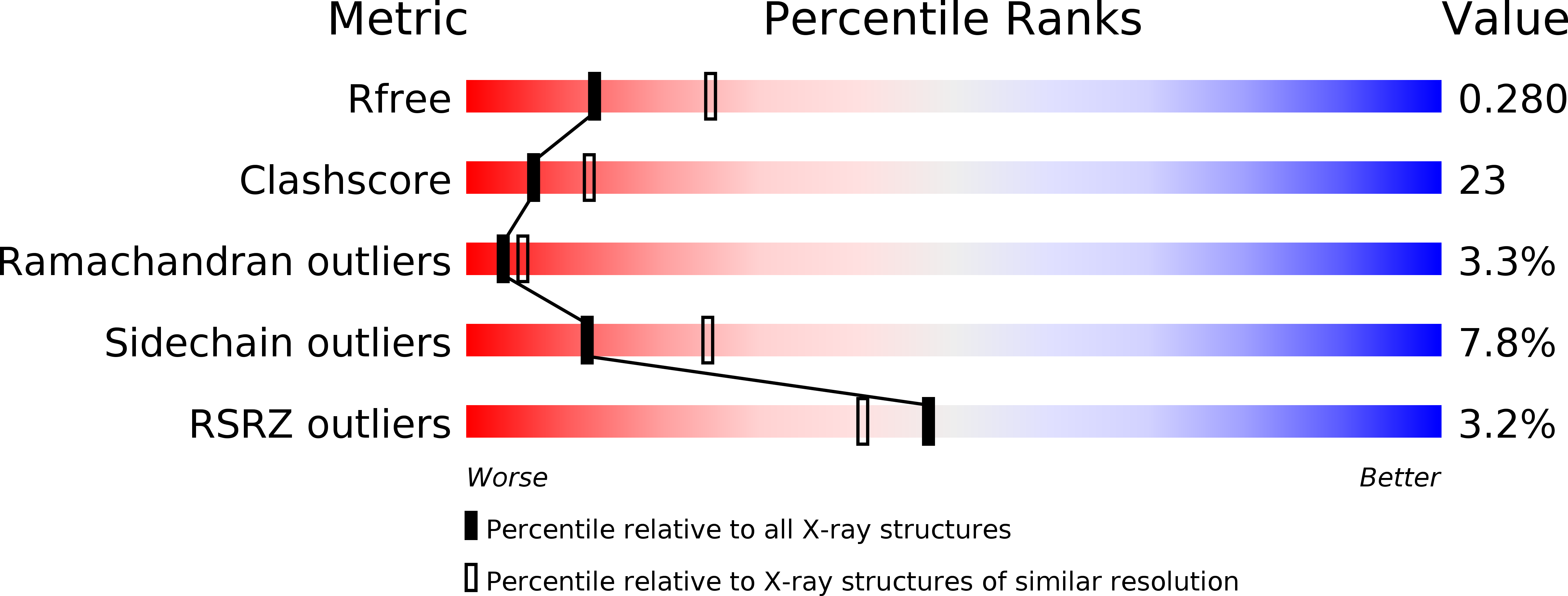
Resolution:
2.60 Å
R-Value Free:
0.28
R-Value Work:
0.24
Space Group:
P 21 21 2