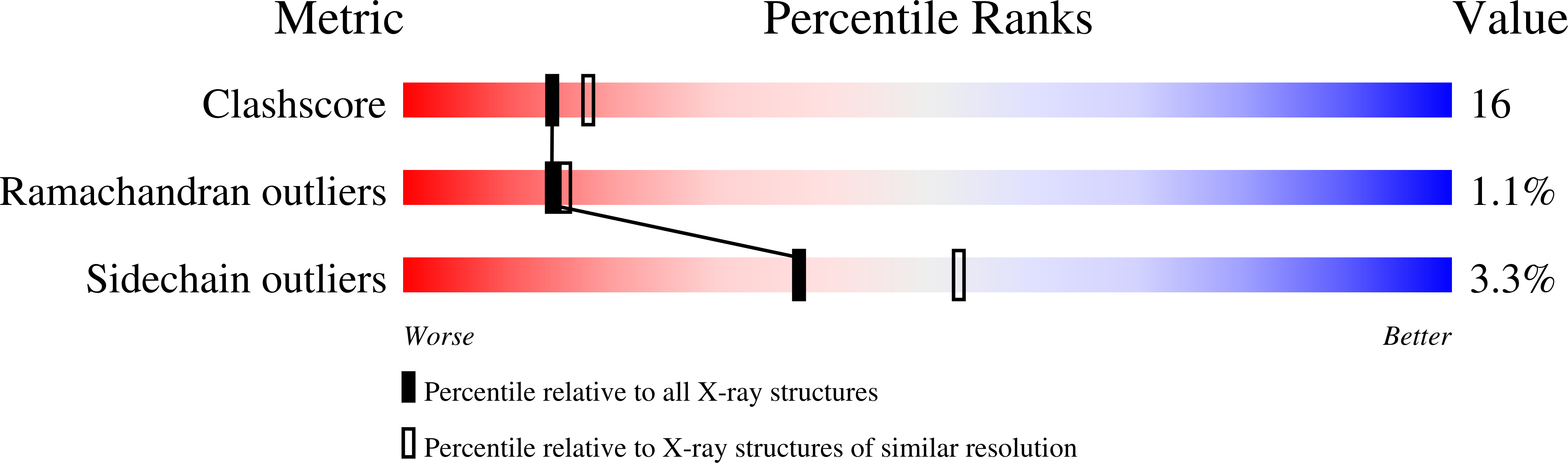Deposition Date
2004-10-29
Release Date
2005-04-12
Last Version Date
2024-11-13
Entry Detail
PDB ID:
1XW7
Keywords:
Title:
Diabetes-Associated Mutations in Human Insulin: Crystal Structure and Photo-Cross-Linking Studies of A-Chain Variant Insulin Wakayama
Method Details:
Experimental Method:
Resolution:
2.30 Å
R-Value Free:
0.26
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
P 21 3