Deposition Date
2004-10-06
Release Date
2005-01-04
Last Version Date
2024-02-14
Entry Detail
PDB ID:
1XOK
Keywords:
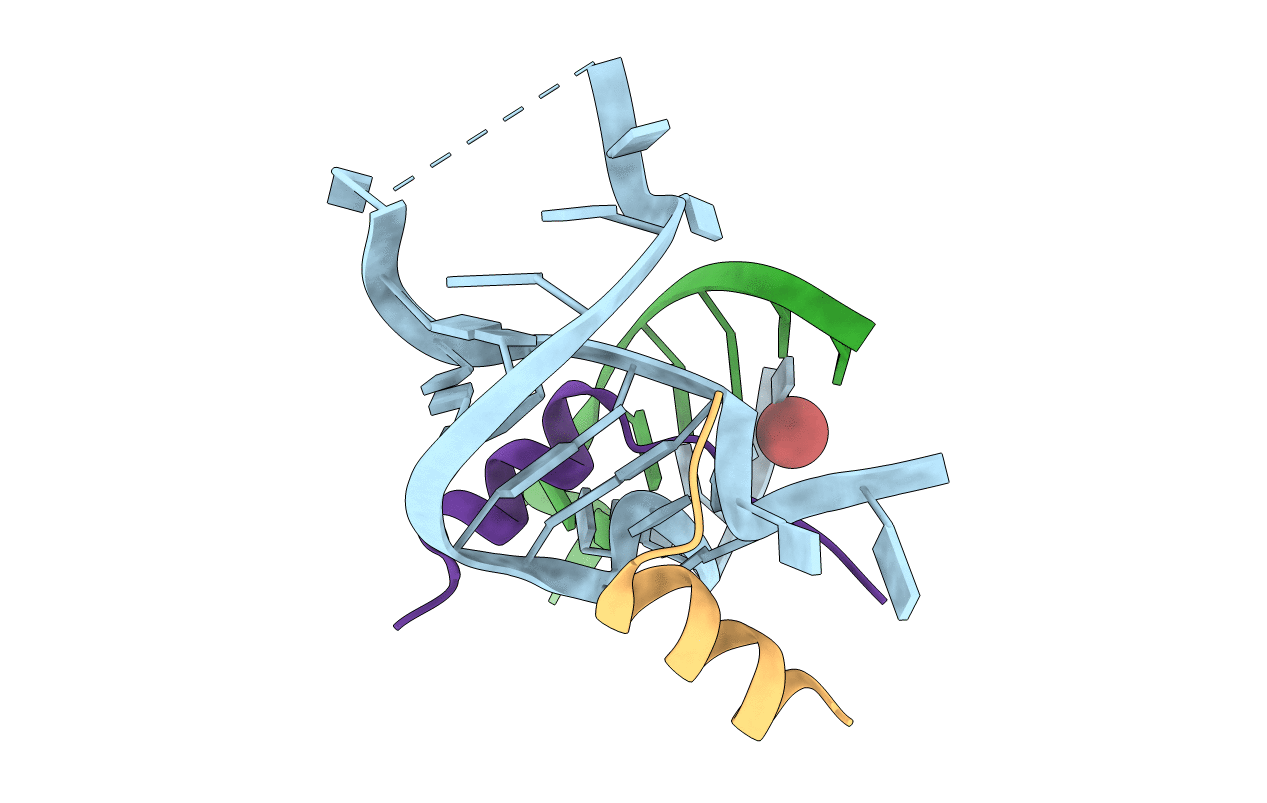
Title:
crystal structure of alfalfa mosaic virus RNA 3'UTR in complex with coat protein N terminal peptide
Method Details:
Experimental Method:
Resolution:
3.00 Å
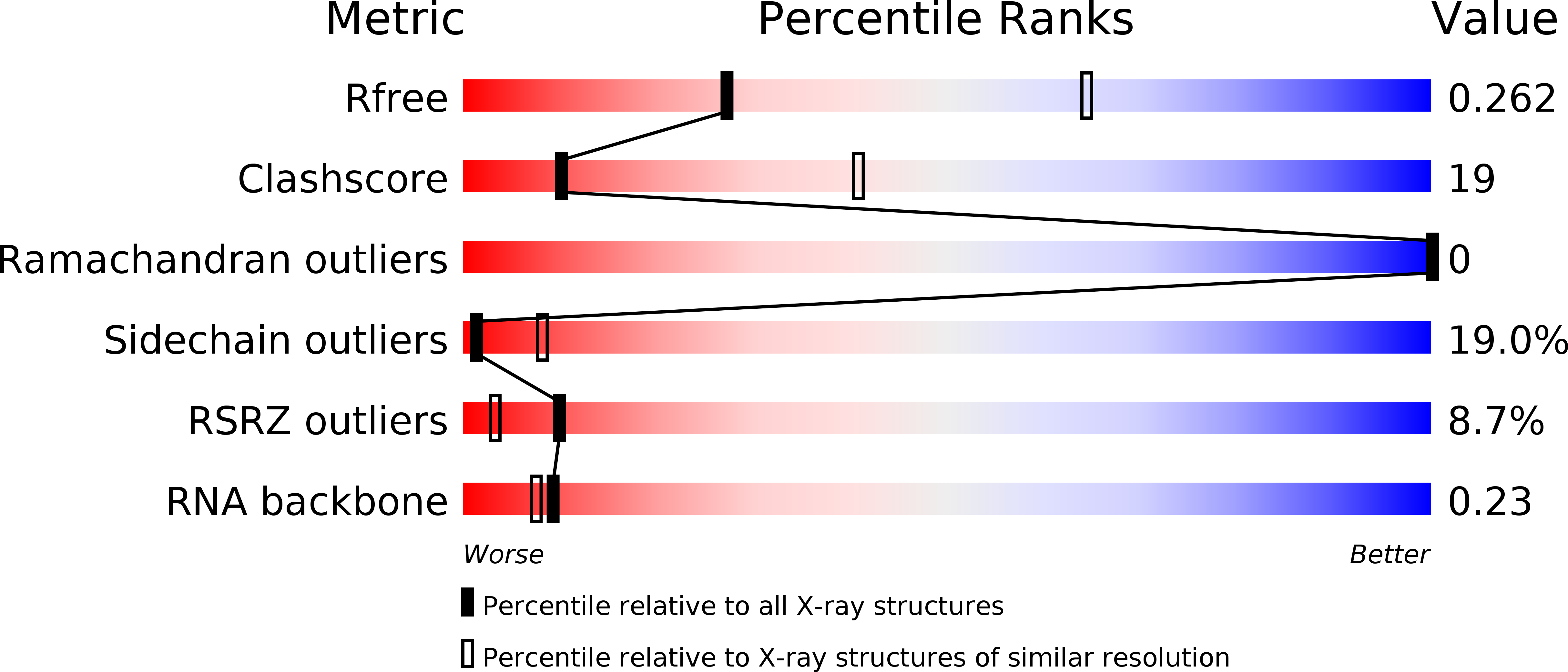
R-Value Free:
0.26
R-Value Work:
0.24
R-Value Observed:
0.25
Space Group:
C 2 2 21