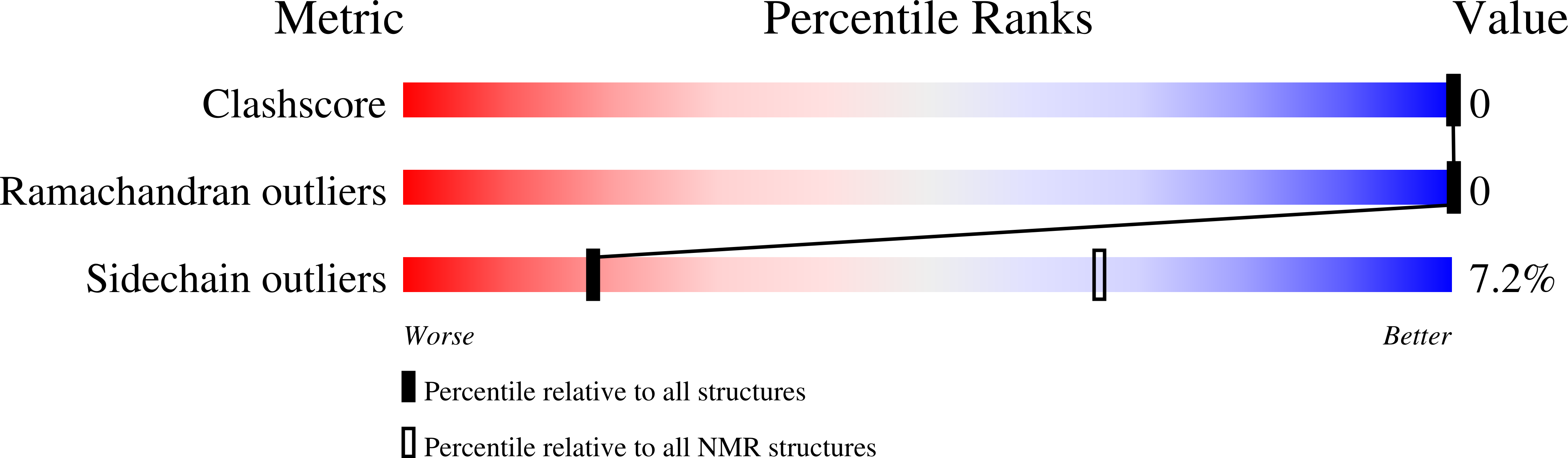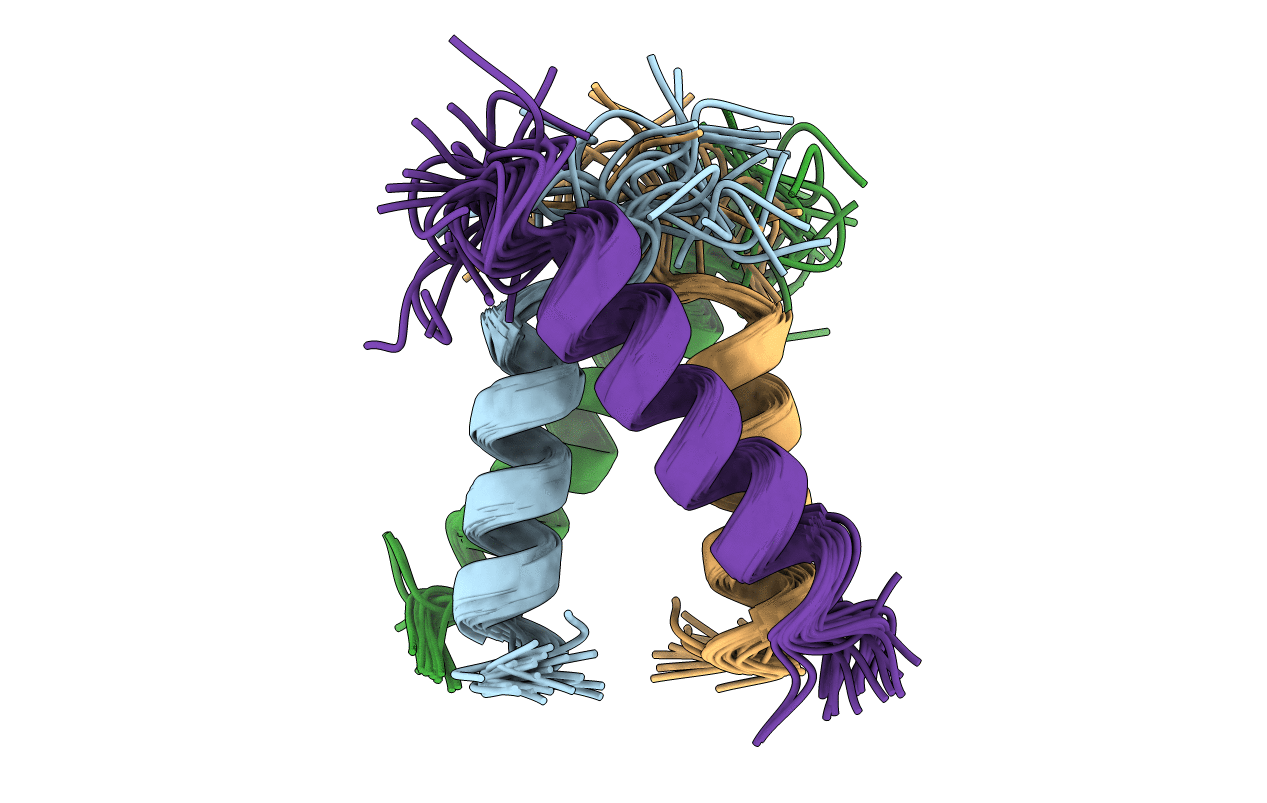
Deposition Date
2004-09-29
Release Date
2005-04-05
Last Version Date
2024-10-23
Entry Detail
Method Details:
Experimental Method:
Conformers Calculated:
150
Conformers Submitted:
24
Selection Criteria:
Structures within a prefixed threshold of amber energy, solvent accessible surface area and symmetry-based penalty functions (see jrnl)