Deposition Date
2004-08-31
Release Date
2004-09-28
Last Version Date
2024-05-22
Entry Detail
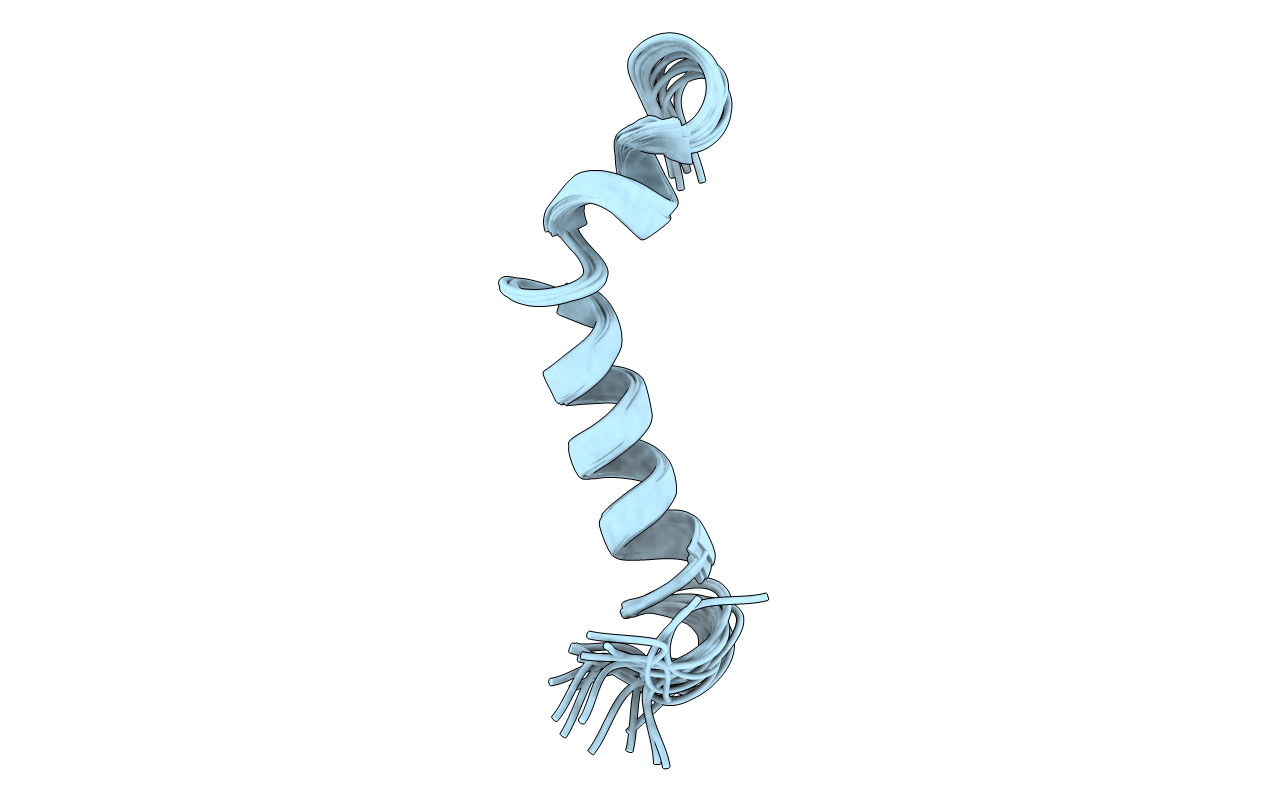
PDB ID:
1XC0
Keywords:
Title:
Twenty Lowest Energy Structures of Pa4 by Solution NMR
Method Details:
Experimental Method:
Conformers Calculated:
350
Conformers Submitted:
20
Selection Criteria:
structures with the lowest energy


