Deposition Date
2004-08-24
Release Date
2004-10-26
Last Version Date
2024-02-14
Entry Detail
PDB ID:
1X9W
Keywords:
Title:
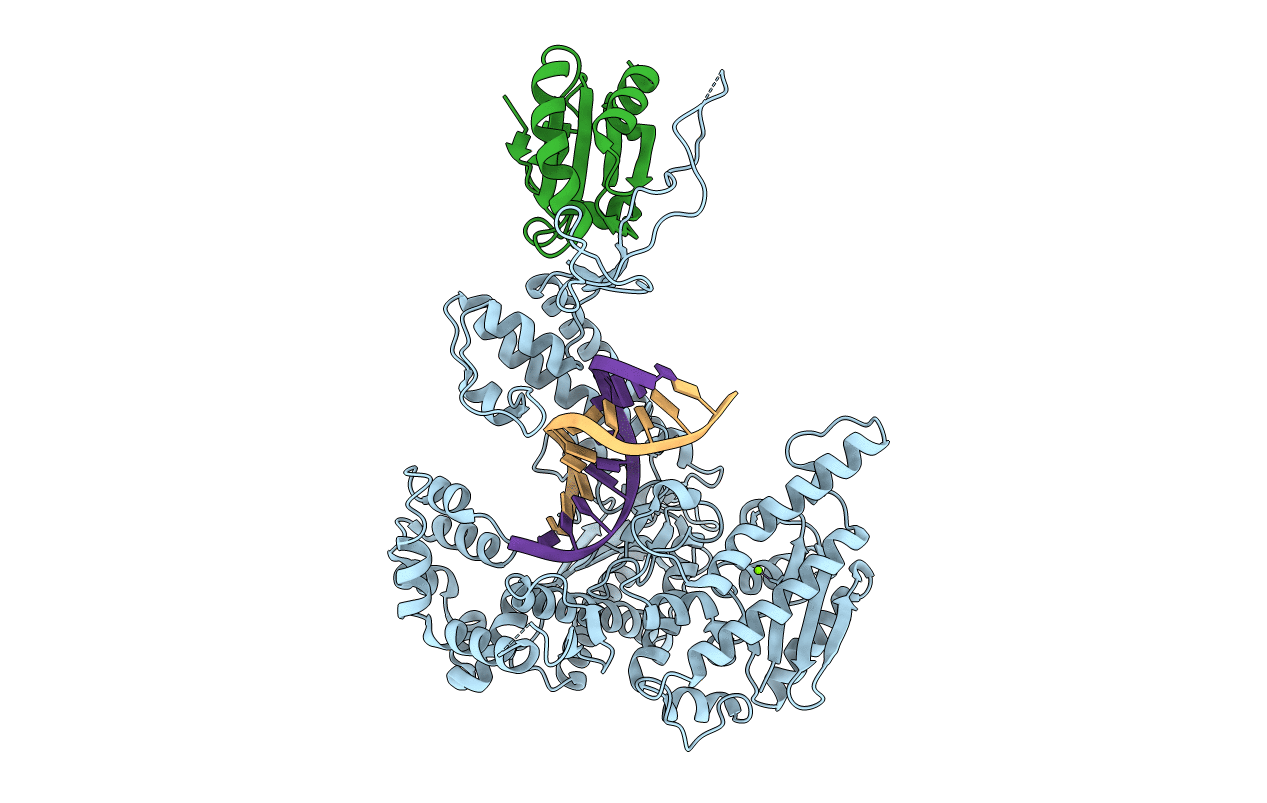
T7 DNA polymerase in complex with a primer/template DNA containing a disordered N-2 aminofluorene on the template, crystallized with dideoxy-ATP as the incoming nucleotide.
Biological Source:
Source Organism(s):
Enterobacteria phage T7 (Taxon ID: 10760)
Escherichia coli (Taxon ID: 562)
Escherichia coli (Taxon ID: 562)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.30 Å
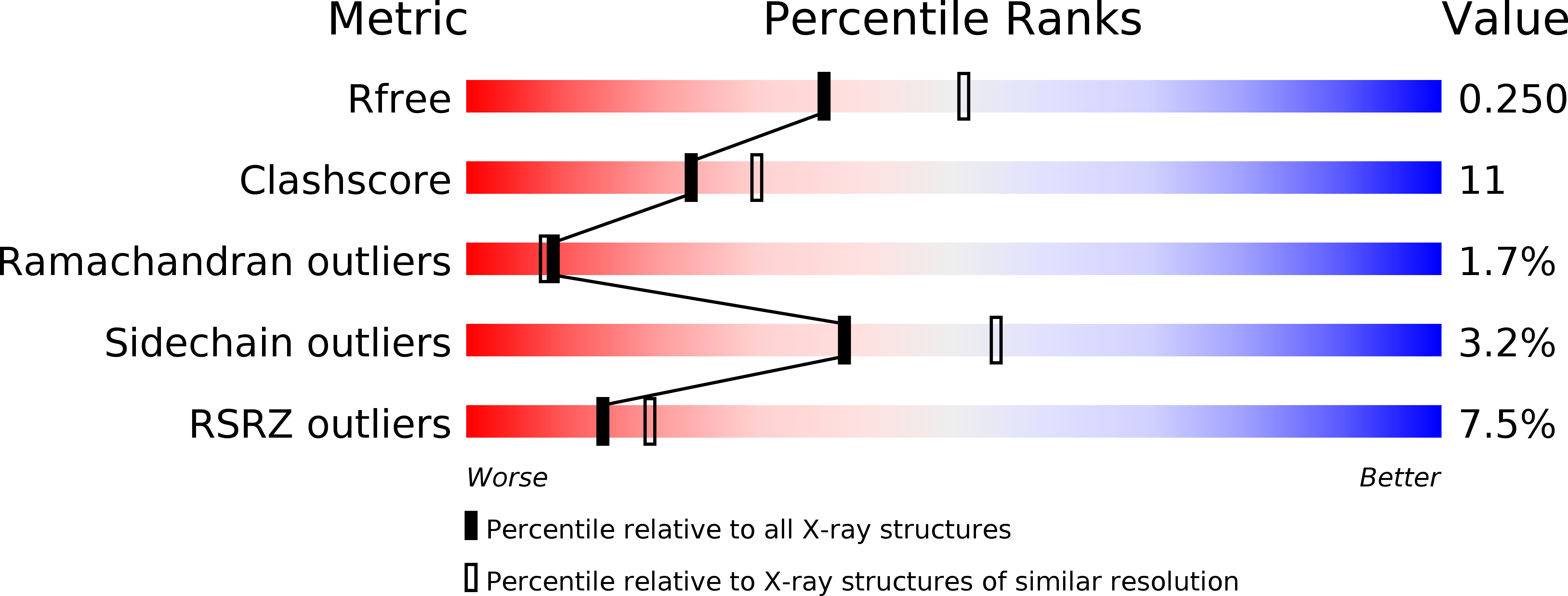
R-Value Free:
0.25
R-Value Work:
0.23
R-Value Observed:
0.23
Space Group:
P 21 21 2