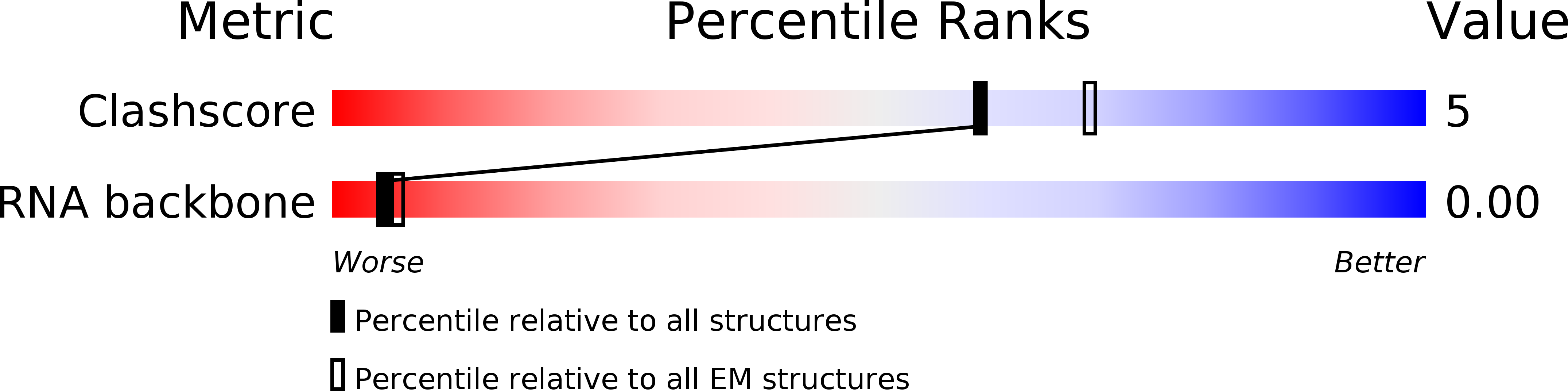Deposition Date
2005-04-06
Release Date
2005-05-17
Last Version Date
2024-03-13
Entry Detail
PDB ID:
1X1L
Keywords:
Title:
Interaction of ERA,a GTPase protein, with the 3'minor domain of the 16S rRNA within the THERMUS THERMOPHILUS 30S subunit.
Biological Source:
Source Organism(s):
Thermus thermophilus (Taxon ID: 274)
Escherichia coli (Taxon ID: 562)
Escherichia coli (Taxon ID: 562)
Method Details:
Experimental Method:
Resolution:
13.50 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE