Deposition Date
2005-04-03
Release Date
2006-07-18
Last Version Date
2023-10-25
Entry Detail
PDB ID:
1X1B
Keywords:
Title:
Crystal structure of BchU complexed with S-adenosyl-L-homocysteine
Biological Source:
Source Organism(s):
Chlorobium tepidum (Taxon ID: 194439)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.60 Å
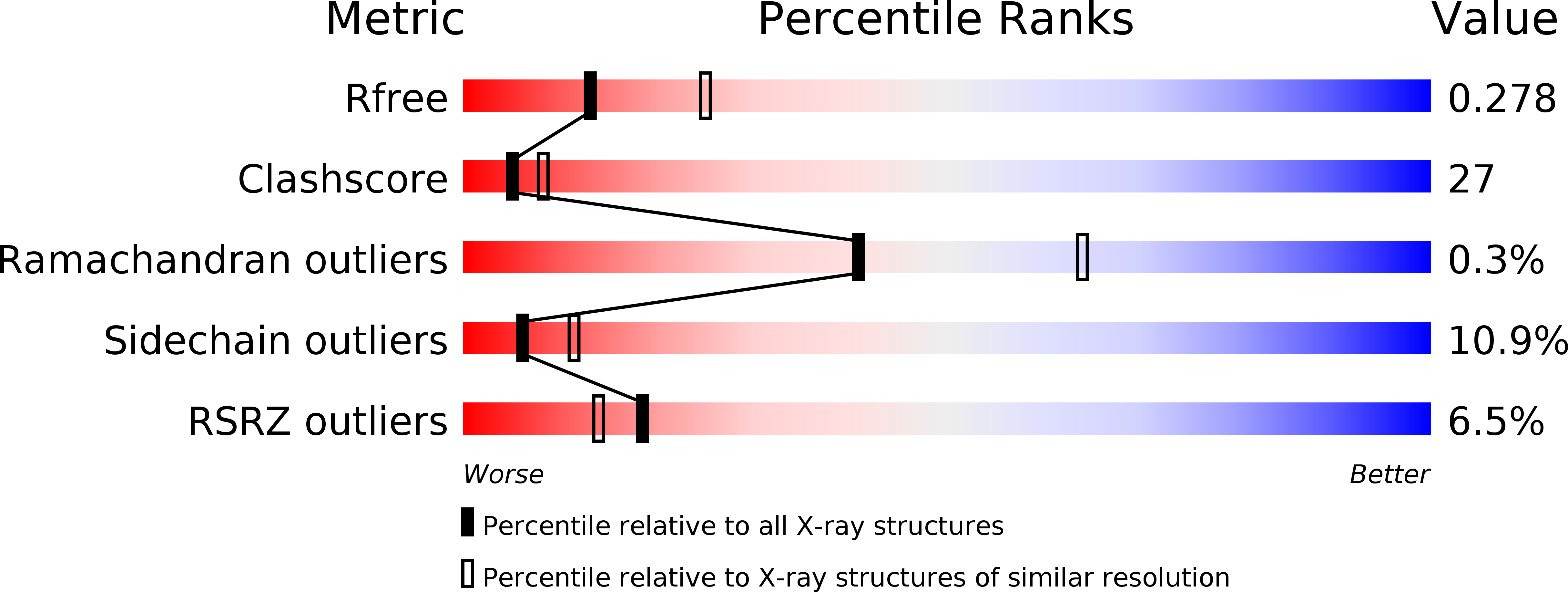
R-Value Free:
0.28
R-Value Work:
0.23
R-Value Observed:
0.23
Space Group:
P 65 2 2