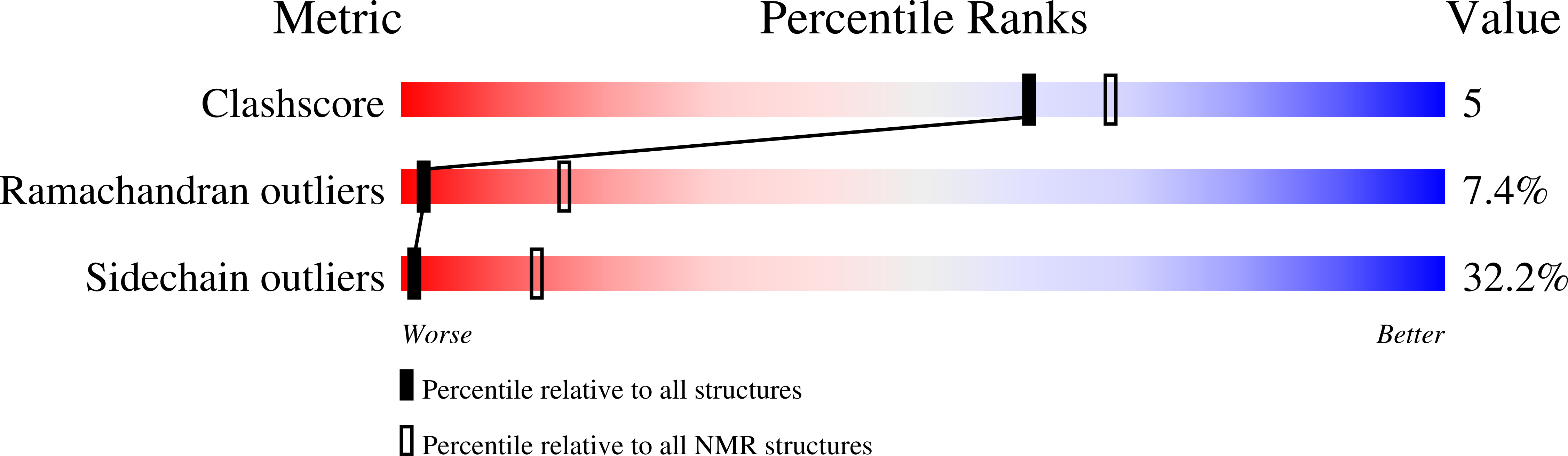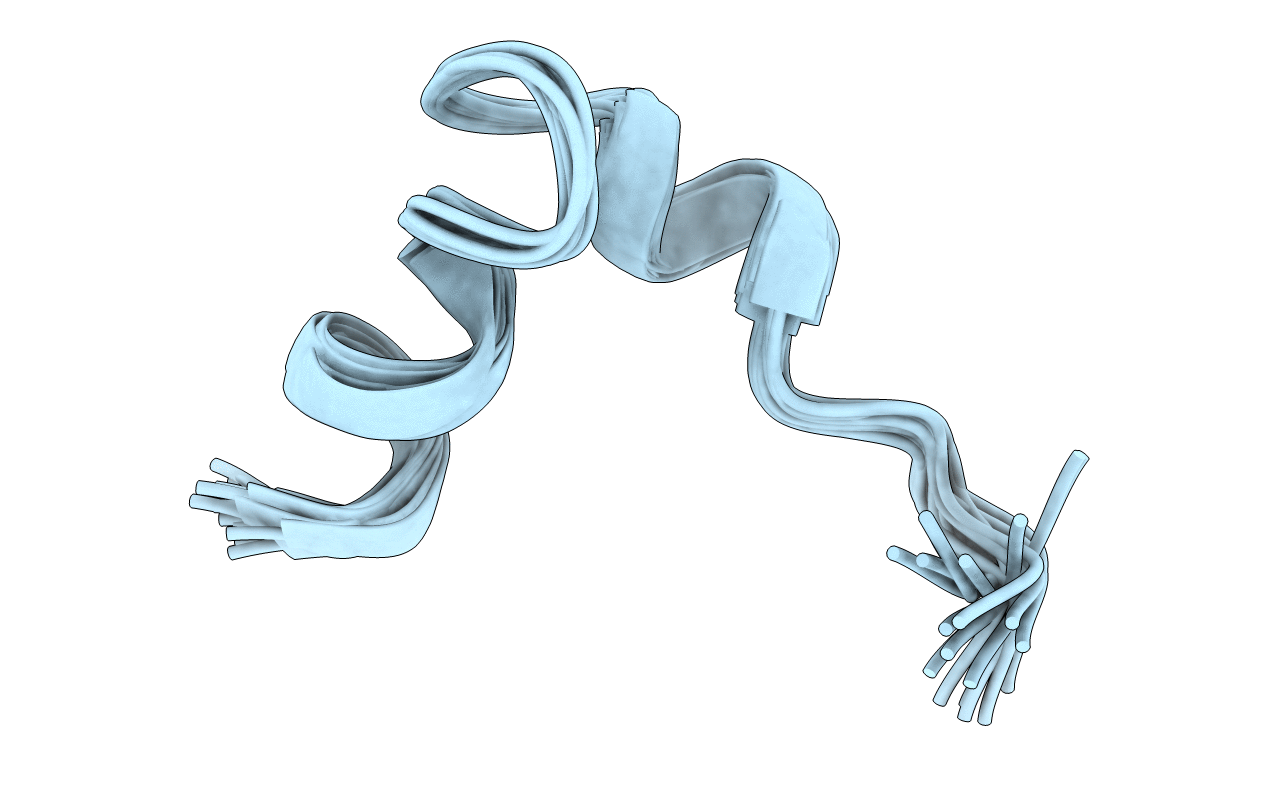
Deposition Date
2005-02-23
Release Date
2006-05-16
Last Version Date
2024-05-29
Entry Detail
PDB ID:
1WZ4
Keywords:
Title:
Solution Conformation of adr subtype HBV Pre-S2 Epitope
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
20
Selection Criteria:
back calculated data agree with experimental NOESY spectrum