Deposition Date
2004-07-08
Release Date
2004-09-14
Last Version Date
2025-03-26
Entry Detail
PDB ID:
1WMF
Keywords:
Title:
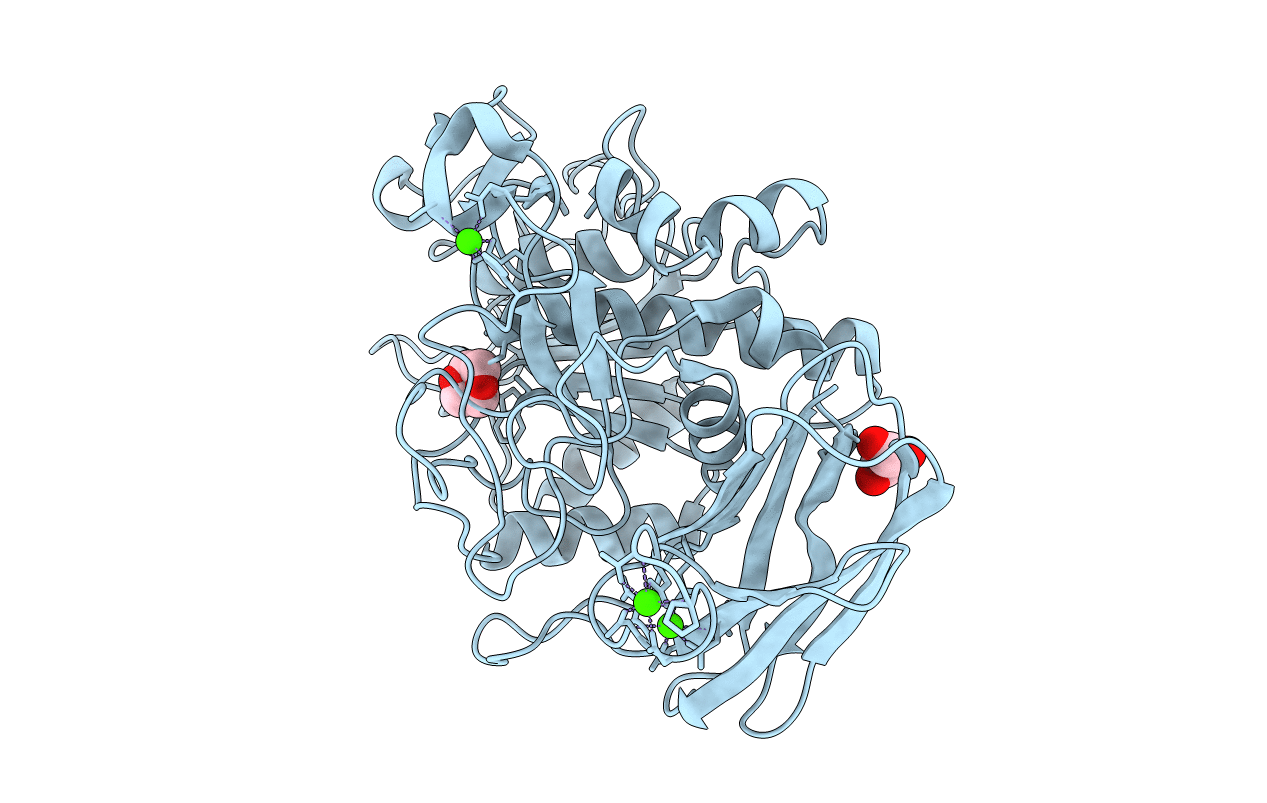
Crystal Structure of alkaline serine protease KP-43 from Bacillus sp. KSM-KP43 (oxidized form, 1.73 angstrom)
Biological Source:
Source Organism(s):
Bacillus sp. (Taxon ID: 109322)
Method Details:
Experimental Method:
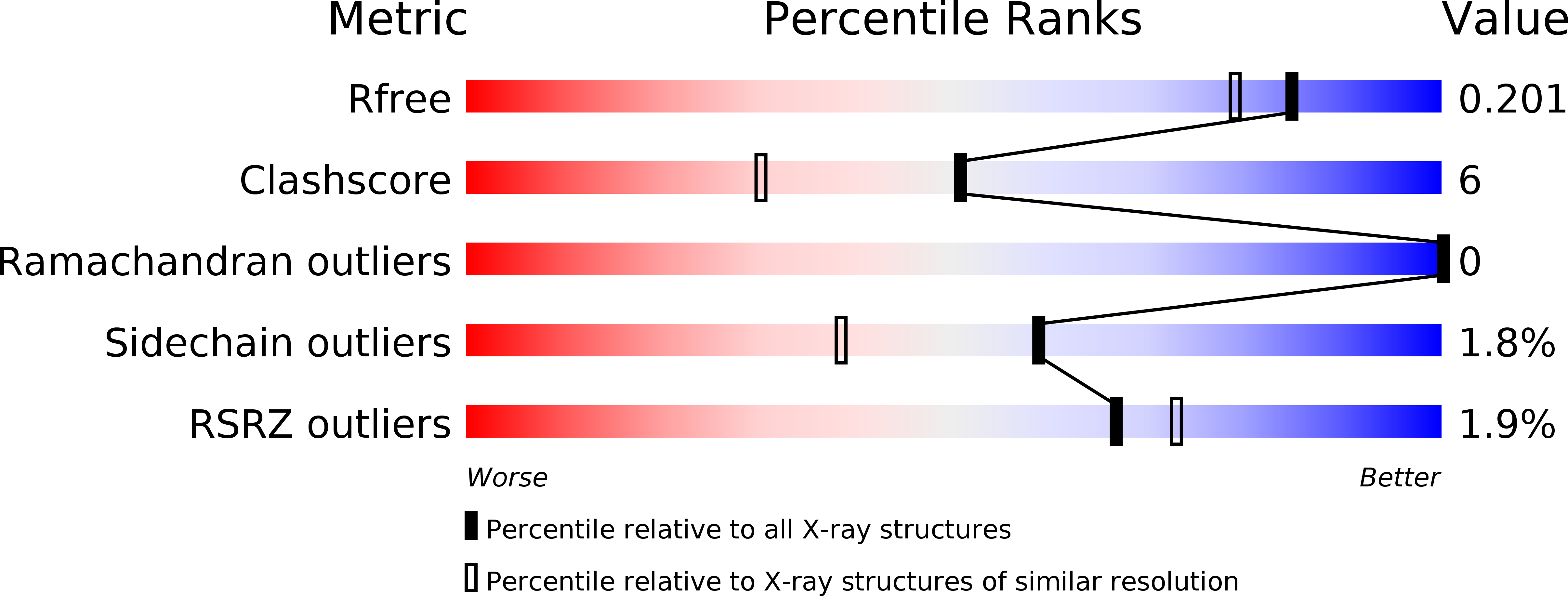
Resolution:
1.73 Å
R-Value Free:
0.21
R-Value Observed:
0.14
Space Group:
C 2 2 21