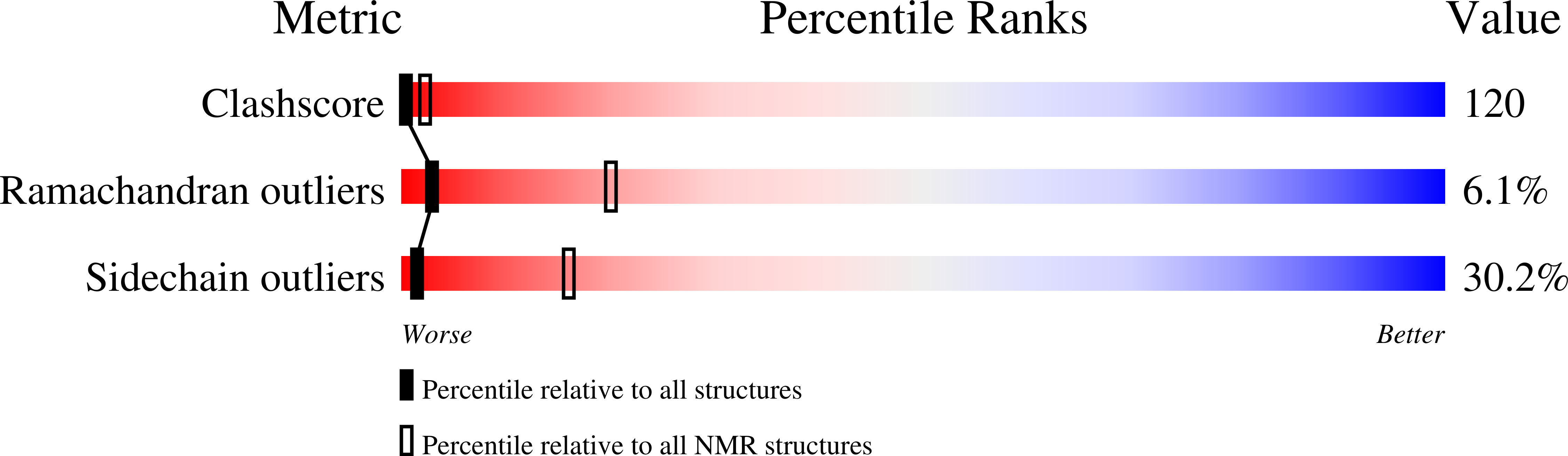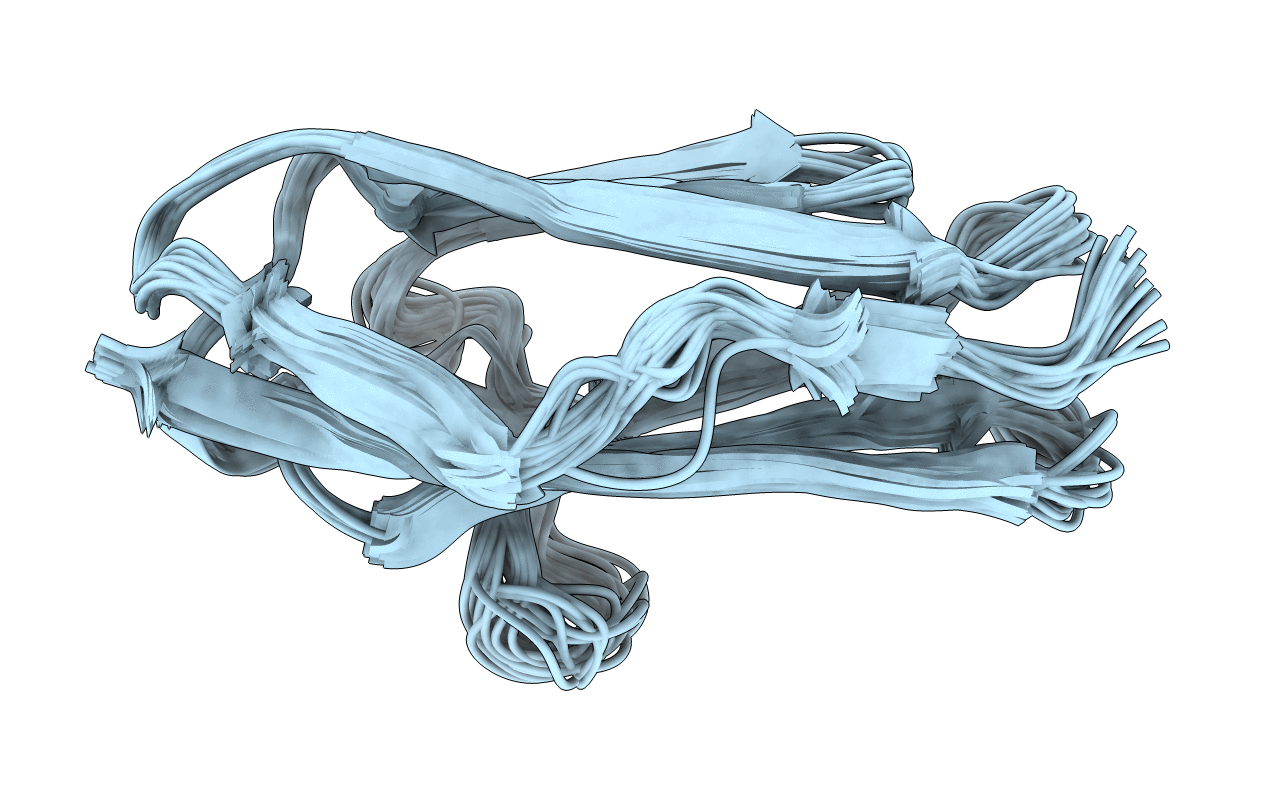
Deposition Date
1996-06-23
Release Date
1996-12-23
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1WIU
Keywords:
Title:
TWITCHIN IMMUNOGLOBULIN SUPERFAMILY DOMAIN (IGSF MODULE) (IG 18'), NMR, 30 STRUCTURES
Biological Source:
Source Organism(s):
Caenorhabditis elegans (Taxon ID: 6239)
Expression System(s):
Method Details:
Experimental Method:
Conformers Submitted:
30