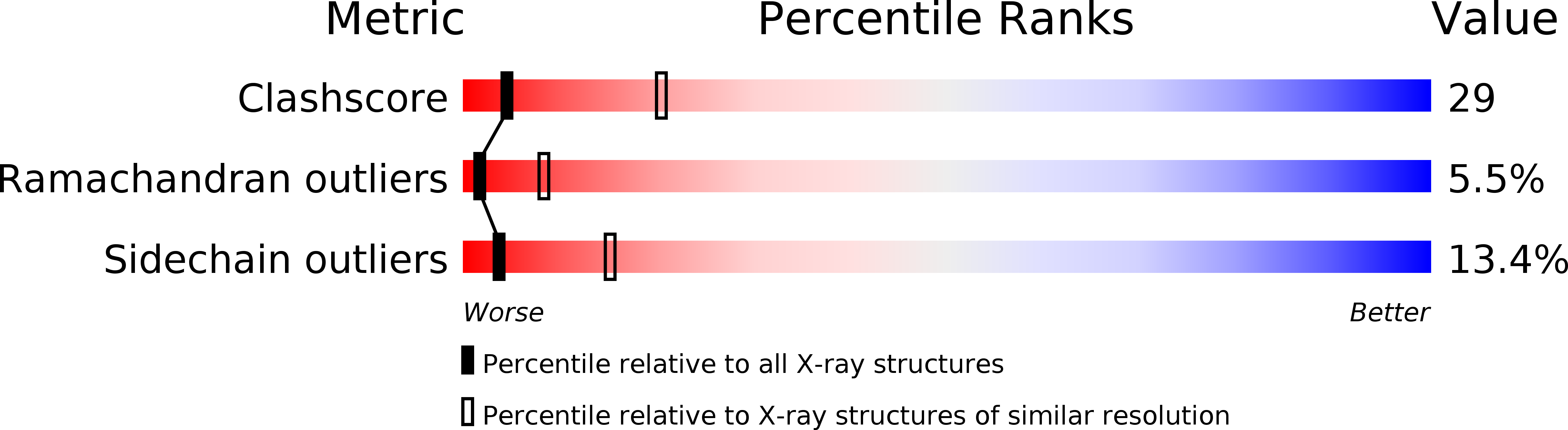
Deposition Date
1995-11-30
Release Date
1996-04-03
Last Version Date
2024-10-16
Entry Detail
PDB ID:
1WBC
Keywords:
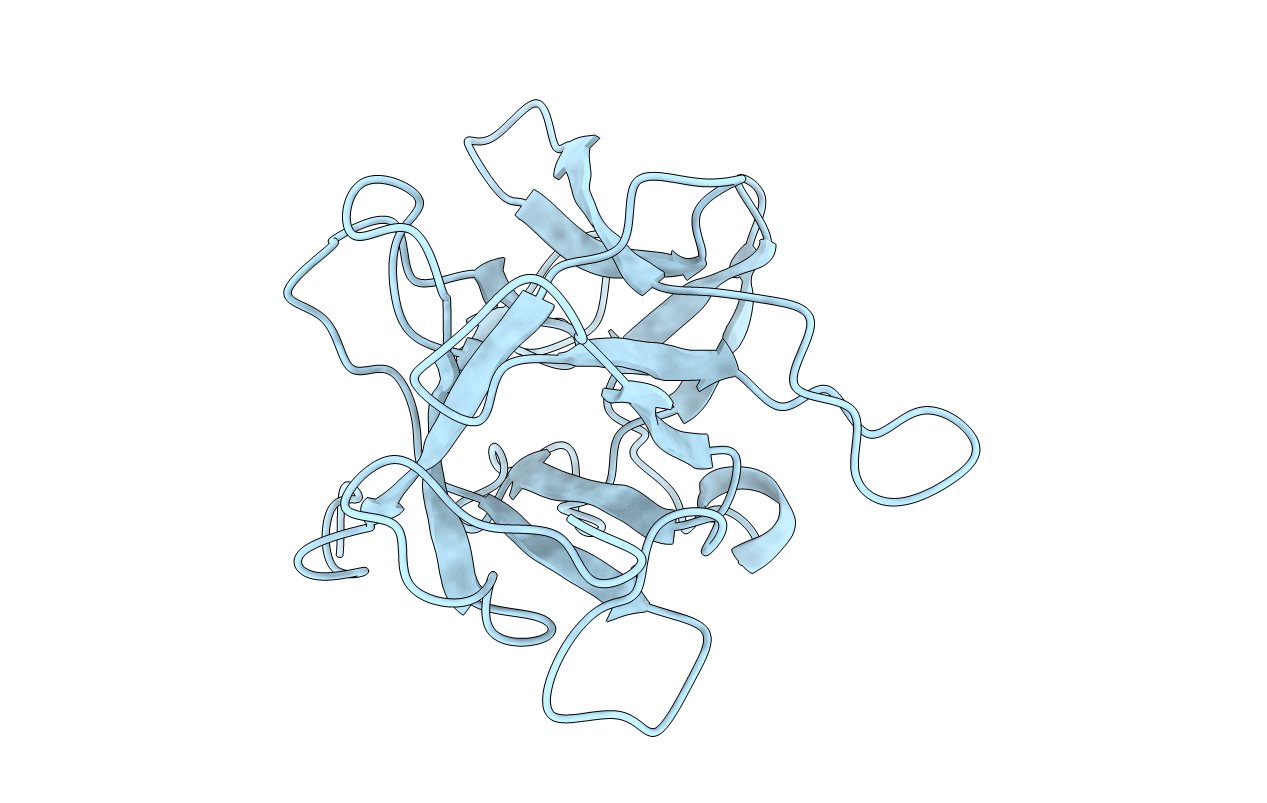
Title:
CRYSTALLIZATION AND PRELIMINARY X-RAY STUDIES OF PSOPHOCARPIN B1, A CHYMOTRYPSIN INHIBITOR FROM WINGED BEAN SEEDS
Biological Source:
Source Organism(s):
Psophocarpus tetragonolobus (Taxon ID: 3891)
Method Details:
Experimental Method:
Resolution:
2.95 Å
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 61 2 2