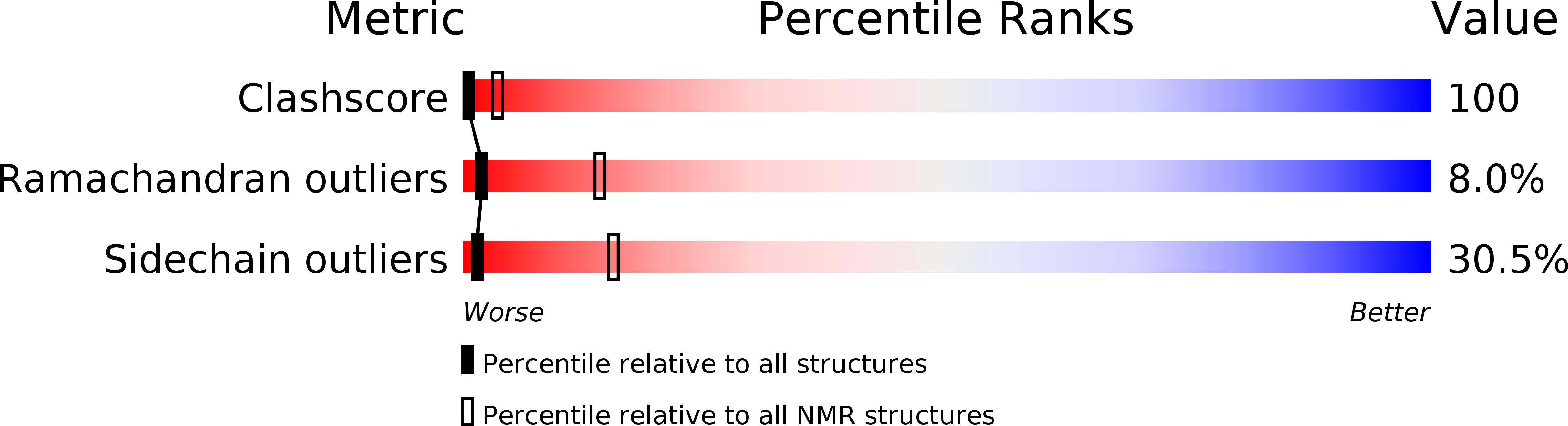Deposition Date
2004-10-25
Release Date
2005-07-07
Last Version Date
2024-05-15
Entry Detail
PDB ID:
1WA7
Keywords:
Title:
SH3 DOMAIN OF HUMAN LYN TYROSINE KINASE IN COMPLEX WITH A HERPESVIRAL LIGAND
Biological Source:
Source Organism(s):
HOMO SAPIENS (Taxon ID: 9606)
SAIMIRIINE HERPESVIRUS 2 (Taxon ID: 10381)
SAIMIRIINE HERPESVIRUS 2 (Taxon ID: 10381)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
60
Conformers Submitted:
20
Selection Criteria:
LEAST RESTRAINT VIOLATION