Deposition Date
2004-09-16
Release Date
2004-11-02
Last Version Date
2023-12-13
Entry Detail
PDB ID:
1W88
Keywords:
Title:
The crystal structure of pyruvate dehydrogenase E1(D180N,E183Q) bound to the peripheral subunit binding domain of E2
Biological Source:
Source Organism(s):
GEOBACILLUS STEAROTHERMOPHILUS (Taxon ID: 1422)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.30 Å
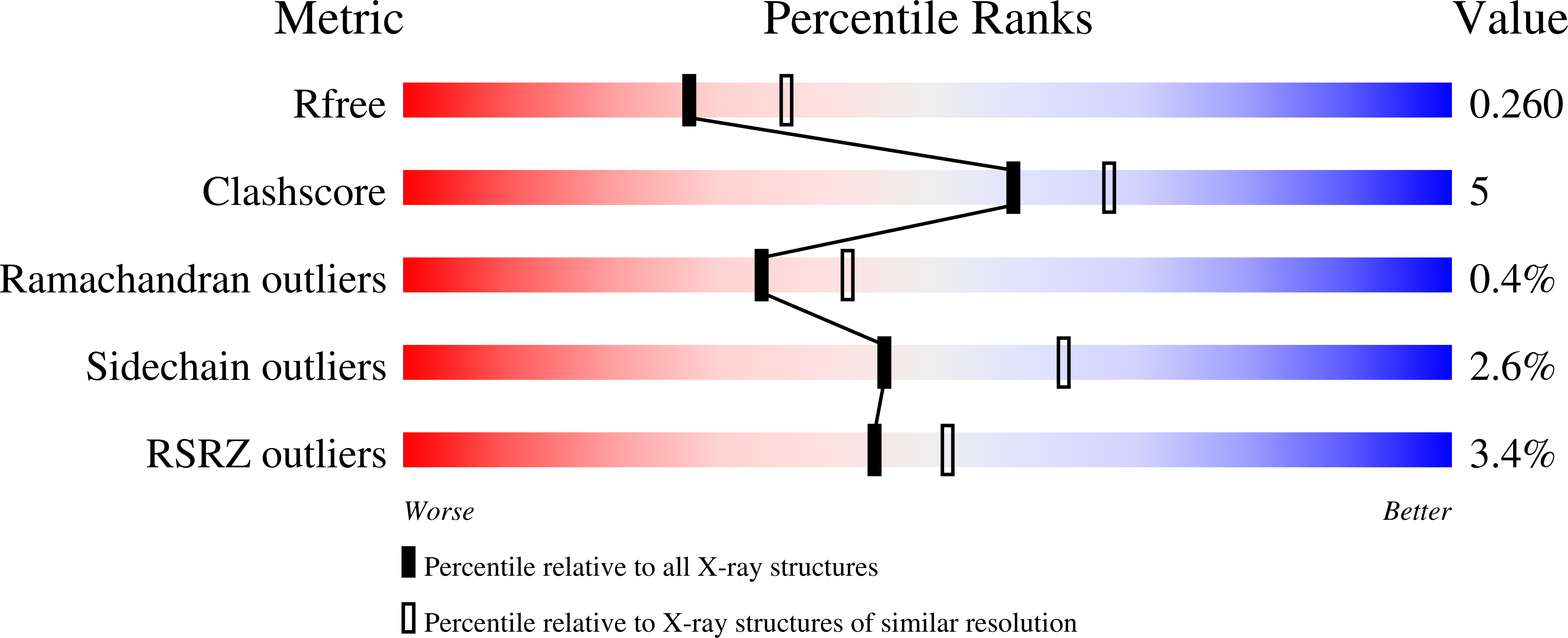
R-Value Free:
0.26
R-Value Work:
0.19
R-Value Observed:
0.20
Space Group:
P 21 21 21