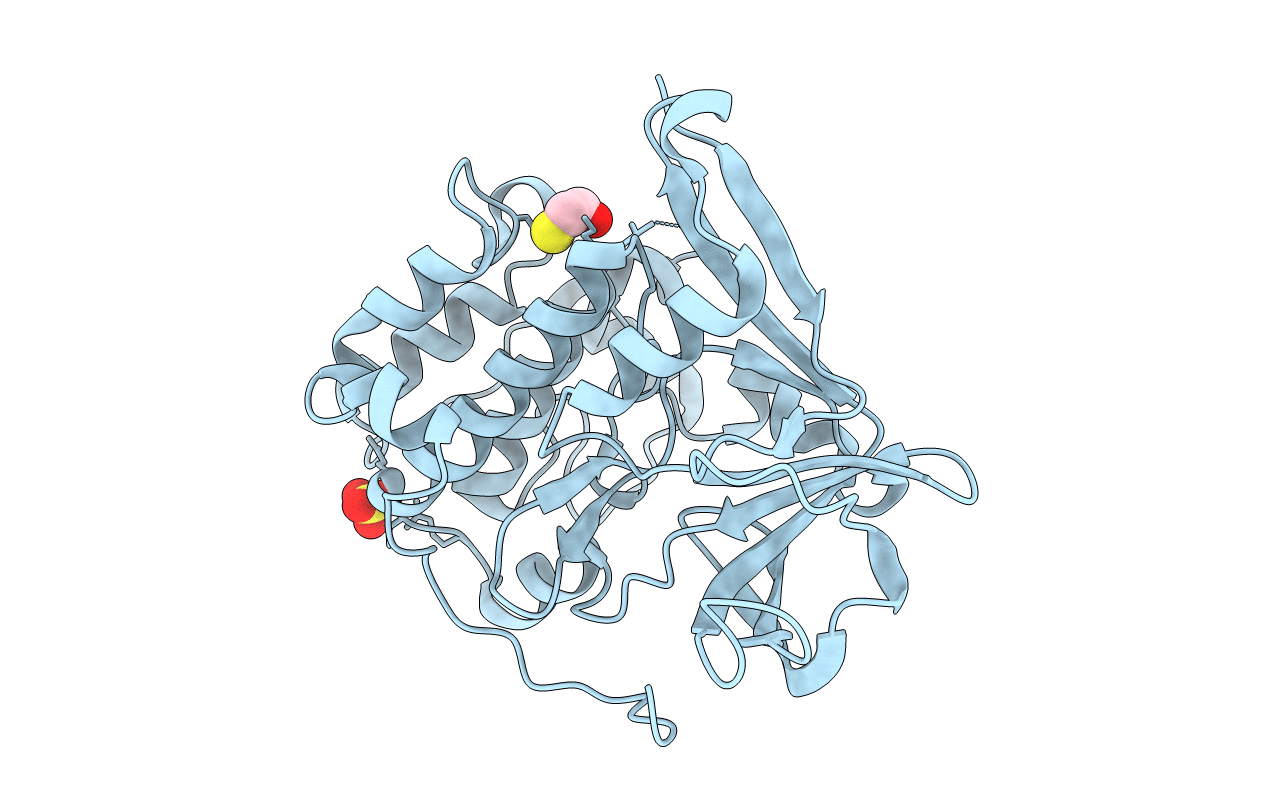
Deposition Date
2004-05-21
Release Date
2004-06-11
Last Version Date
2023-12-13
Entry Detail
PDB ID:
1VZO
Keywords:
Title:
The structure of the N-terminal kinase domain of MSK1 reveals a novel autoinhibitory conformation for a dual kinase protein
Biological Source:
Source Organism:
HOMO SAPIENS (Taxon ID: 9606)
Host Organism:
Method Details:
Experimental Method:
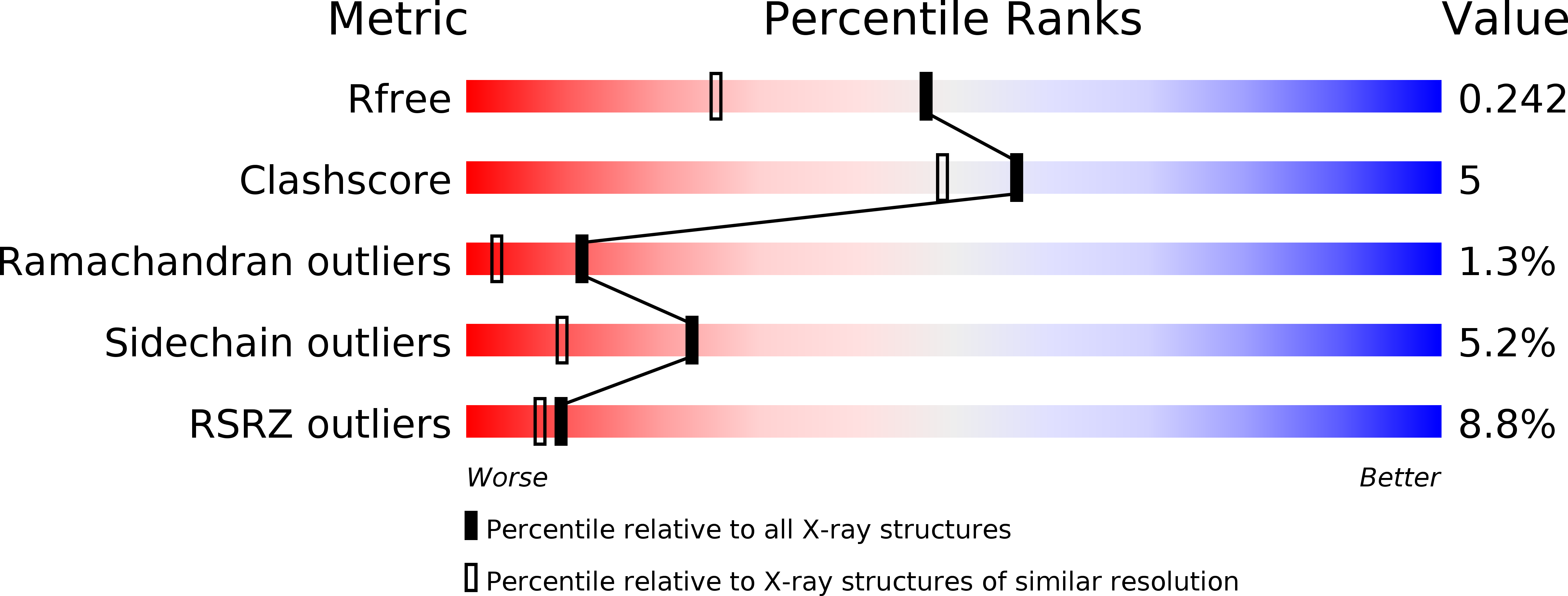
Resolution:
1.80 Å
R-Value Free:
0.24
R-Value Work:
0.20
Space Group:
P 21 21 21