Deposition Date
2014-08-14
Release Date
2015-04-08
Last Version Date
2023-09-27
Entry Detail
PDB ID:
1VYA
Keywords:
Title:
Identification and characterization of the first plant G-quadruplex binding protein encoded by the Zea mays L. nucleoside diphosphate1 gene, ZmNDPK1
Biological Source:
Expression System(s):
Method Details:
Experimental Method:
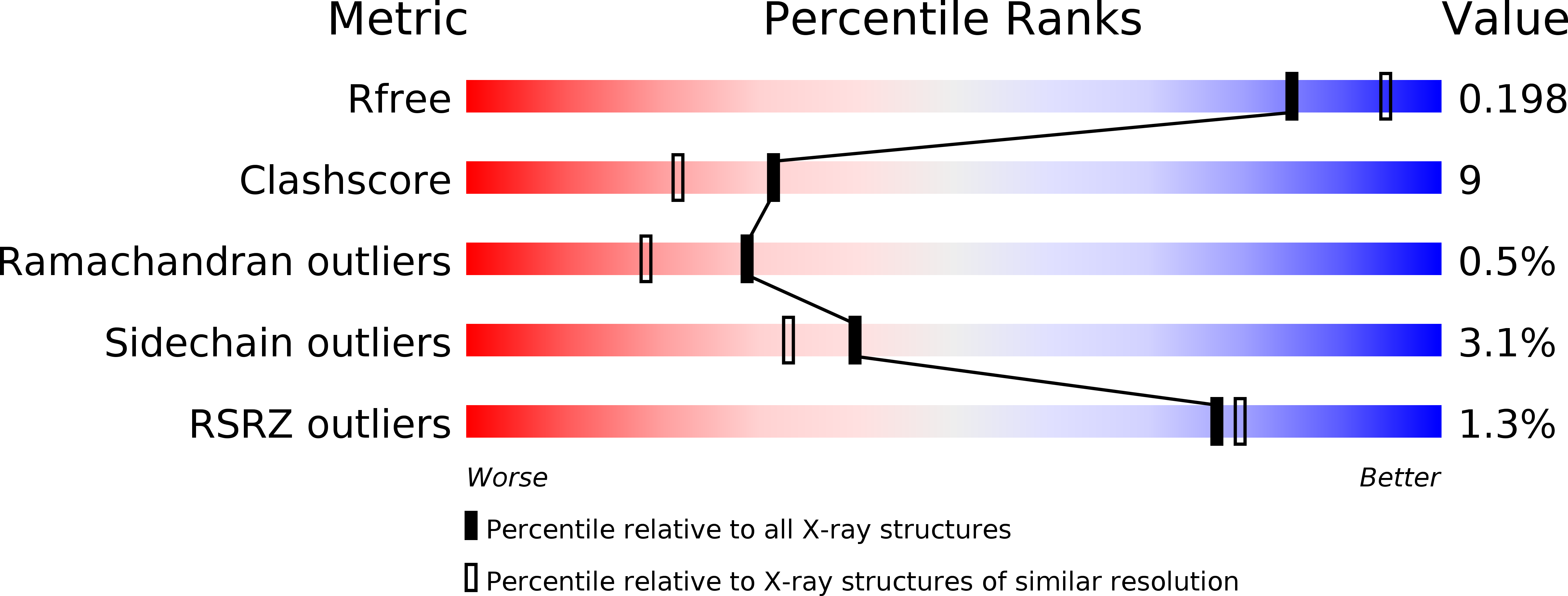
Resolution:
2.05 Å
R-Value Free:
0.20
R-Value Work:
0.16
R-Value Observed:
0.16
Space Group:
P 1 21 1