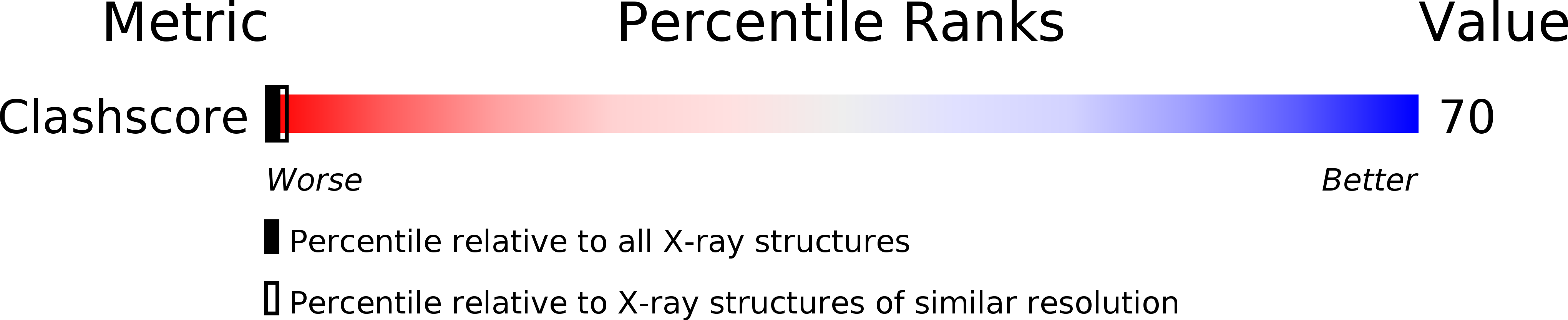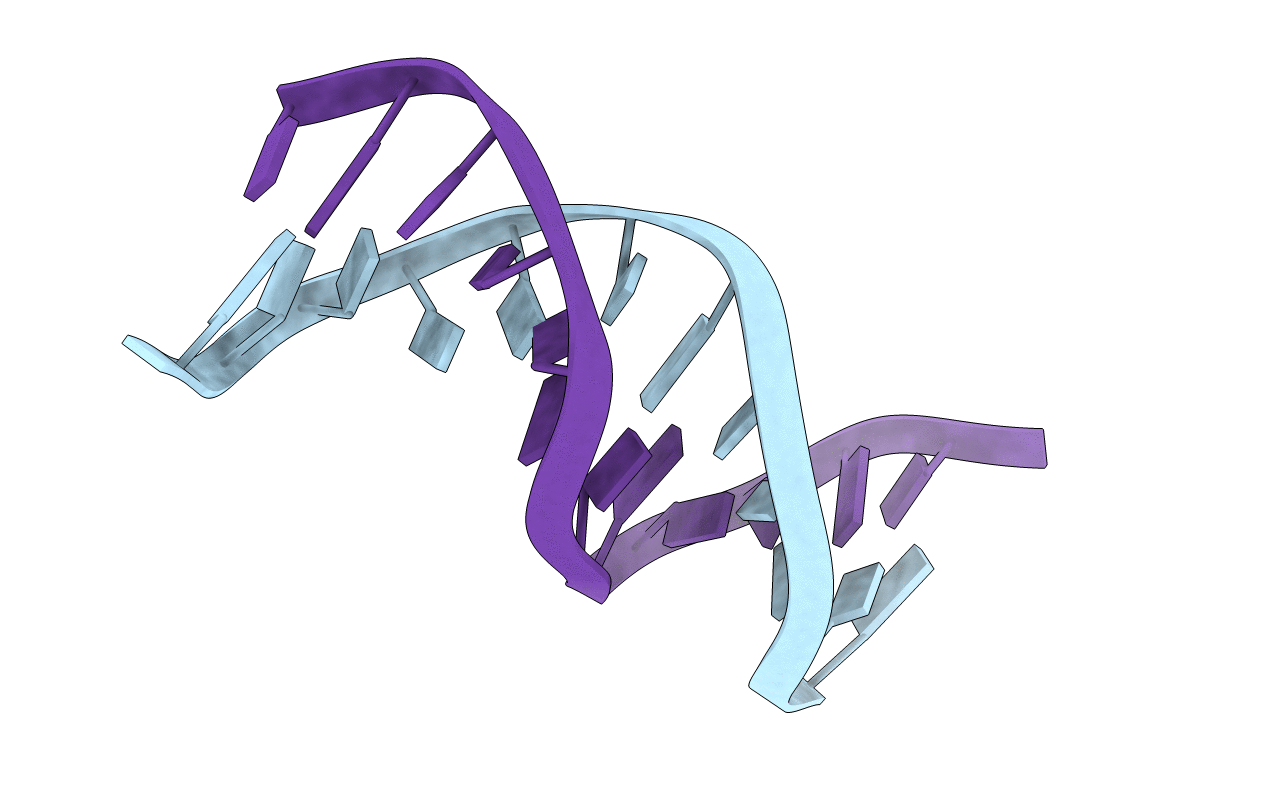
Deposition Date
1996-12-12
Release Date
2011-07-13
Last Version Date
2023-12-27
Entry Detail
PDB ID:
1VTD
Keywords:
Title:
UNUSUAL HELICAL PACKING IN CRYSTALS OF DNA BEARING A MUTATION HOT SPOT
Biological Source:
Source Organism(s):
synthetic construct (Taxon ID: 32630)
Method Details: