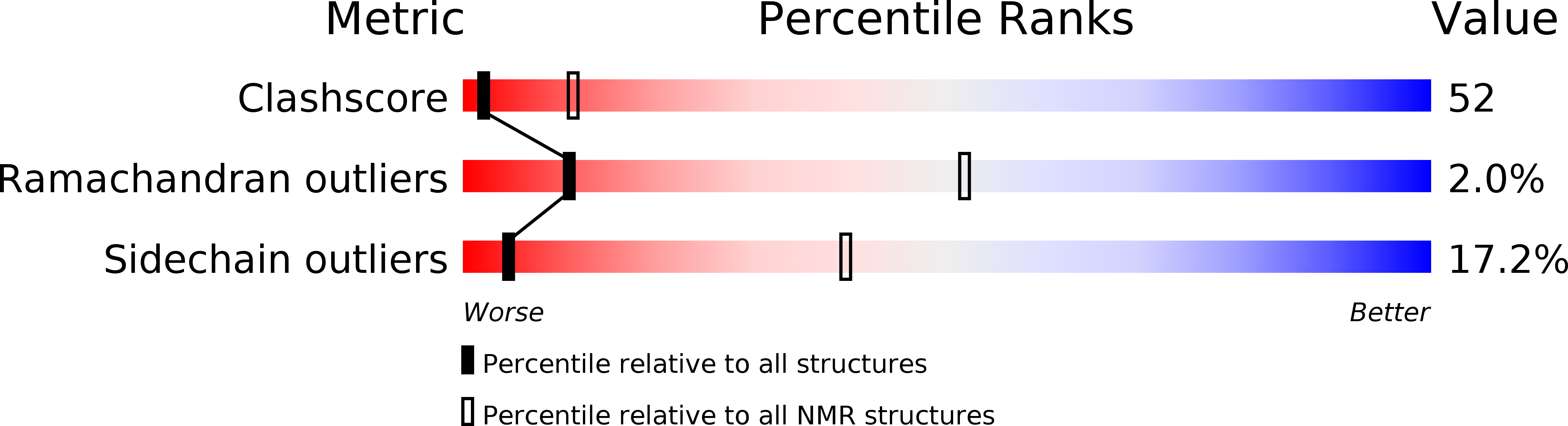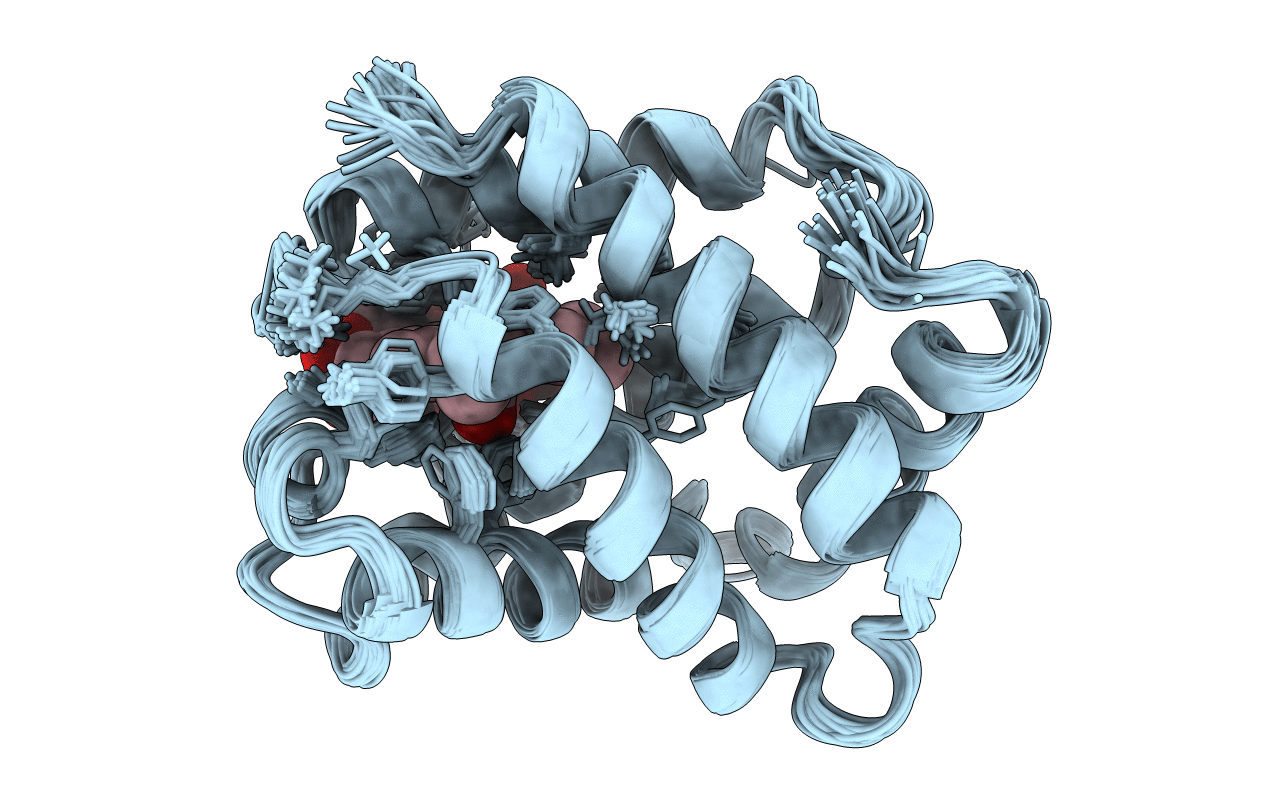
Deposition Date
1999-03-25
Release Date
1999-04-02
Last Version Date
2023-12-27
Entry Detail
PDB ID:
1VRE
Keywords:
Title:
SOLUTION STRUCTURE OF COMPONENT IV GLYCERA DIBRANCHIATA MONOMERIC HEMOGLOBIN-CO
Biological Source:
Source Organism(s):
Glycera dibranchiata (Taxon ID: 6350)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
29
Selection Criteria:
NO NOE VIOLATIONS GREATER THAN 0.2 ANGSTROM, NO DIHEDRAL RESTRAINT VIOLATIONS GREATER THAN 2 DEGREES.