Deposition Date
2004-12-16
Release Date
2005-11-29
Last Version Date
2023-11-15
Entry Detail
PDB ID:
1VQ7
Keywords:
Title:
The structure of the transition state analogue "DCA" bound to the large ribosomal subunit of haloarcula marismortui
Biological Source:
Source Organism(s):
Haloarcula marismortui (Taxon ID: 2238)
Method Details:
Experimental Method:
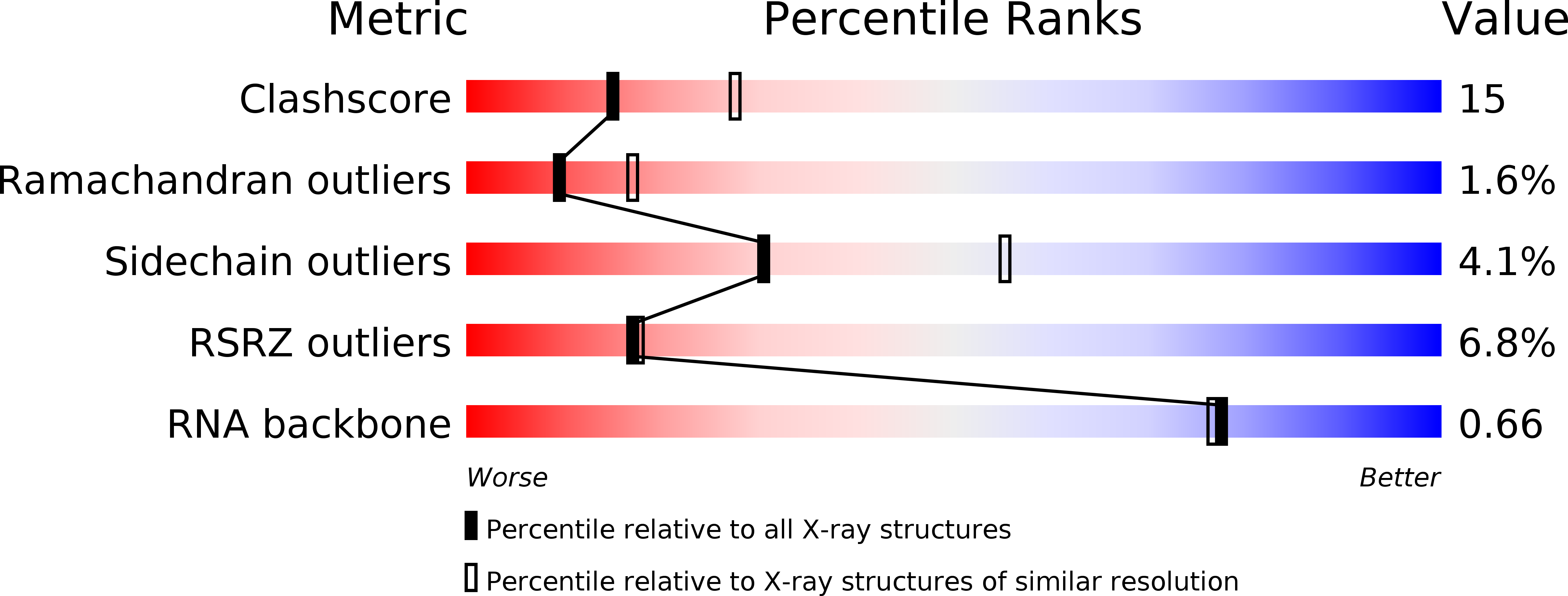
Resolution:
2.50 Å
R-Value Free:
0.24
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
C 2 2 21