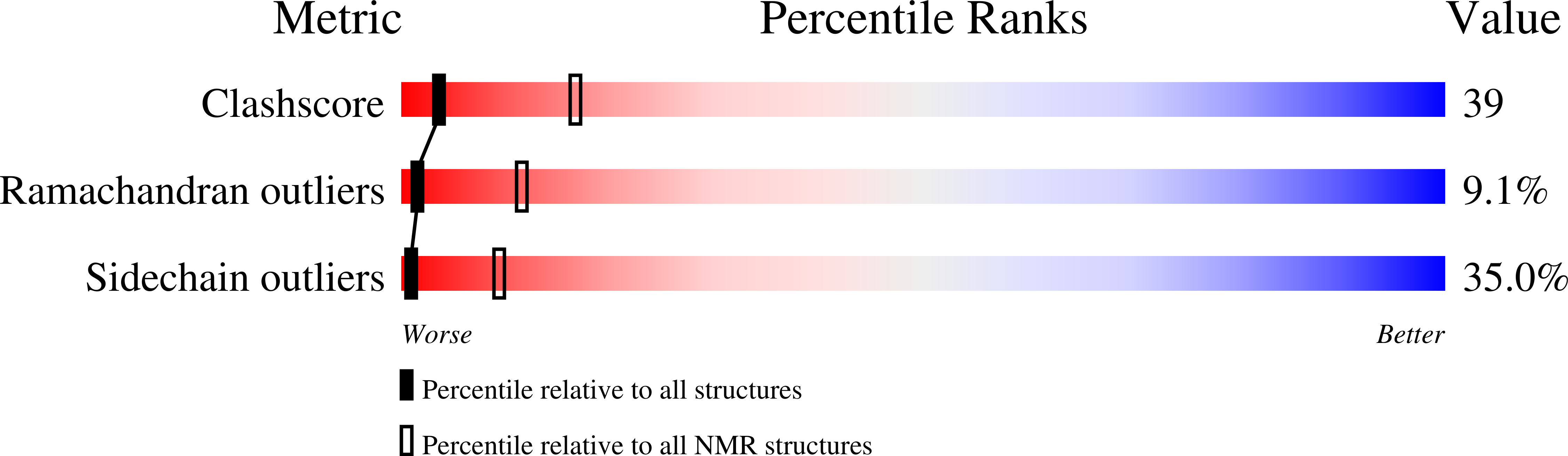
Deposition Date
1993-10-26
Release Date
1994-01-31
Last Version Date
2024-10-09
Entry Detail
PDB ID:
1VNA
Keywords:
Title:
PROTON NUCLEAR MAGNETIC RESONANCE AND DISTANCE GEOMETRY(SLASH)SIMULATED ANNEALING STUDIES ON THE VARIANT-1 NEUROTOXIN FROM THE NEW WORLD SCORPION CENTRUROIDES SCULPTURATUS EWING
Biological Source:
Source Organism(s):
Centruroides sculpturatus (Taxon ID: 218467)
Method Details:
Experimental Method:
Conformers Submitted:
26