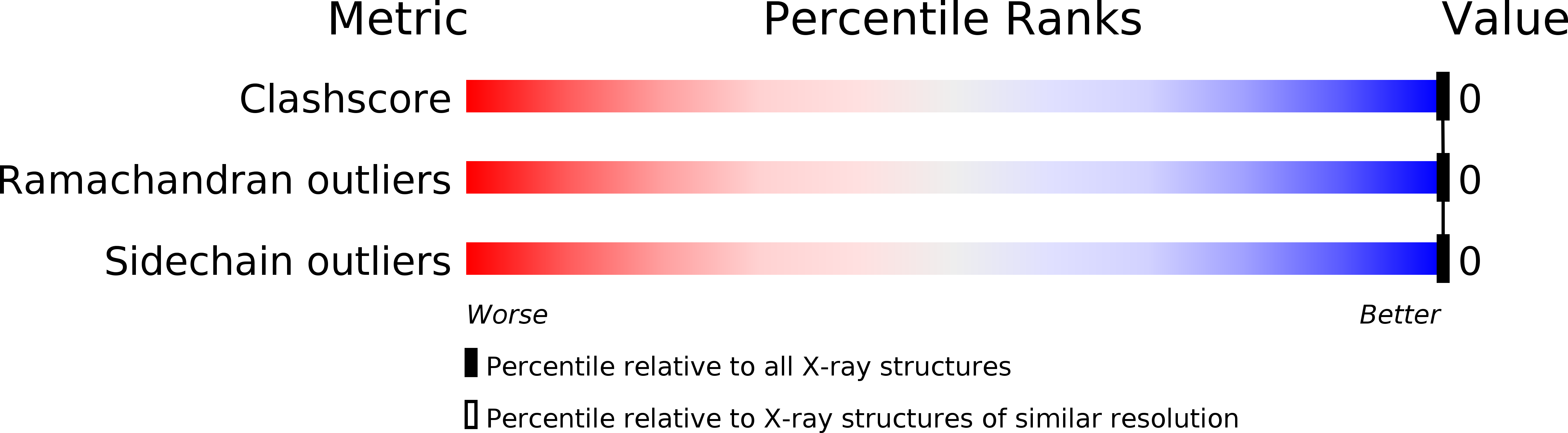
Deposition Date
2003-12-03
Release Date
2004-08-03
Last Version Date
2024-04-03
Entry Detail
PDB ID:
1V6Q
Keywords:
Title:
Crystal Structures of Collagen Model Peptides with Pro-Hyp-Gly Sequence at 1.3 A
Method Details:
Experimental Method:
Resolution:
1.25 Å
R-Value Free:
0.18
R-Value Work:
0.13
Space Group:
P 1 21 1