Deposition Date
2003-11-11
Release Date
2004-08-03
Last Version Date
2023-10-25
Entry Detail
PDB ID:
1V48
Keywords:
Title:
Calf spleen purine nucleoside phosphorylase (PNP) binary complex with 9-(5,5-difluoro-5-phosphonopenthyl)guanine
Biological Source:
Source Organism(s):
Bos taurus (Taxon ID: 9913)
Method Details:
Experimental Method:
Resolution:
2.20 Å
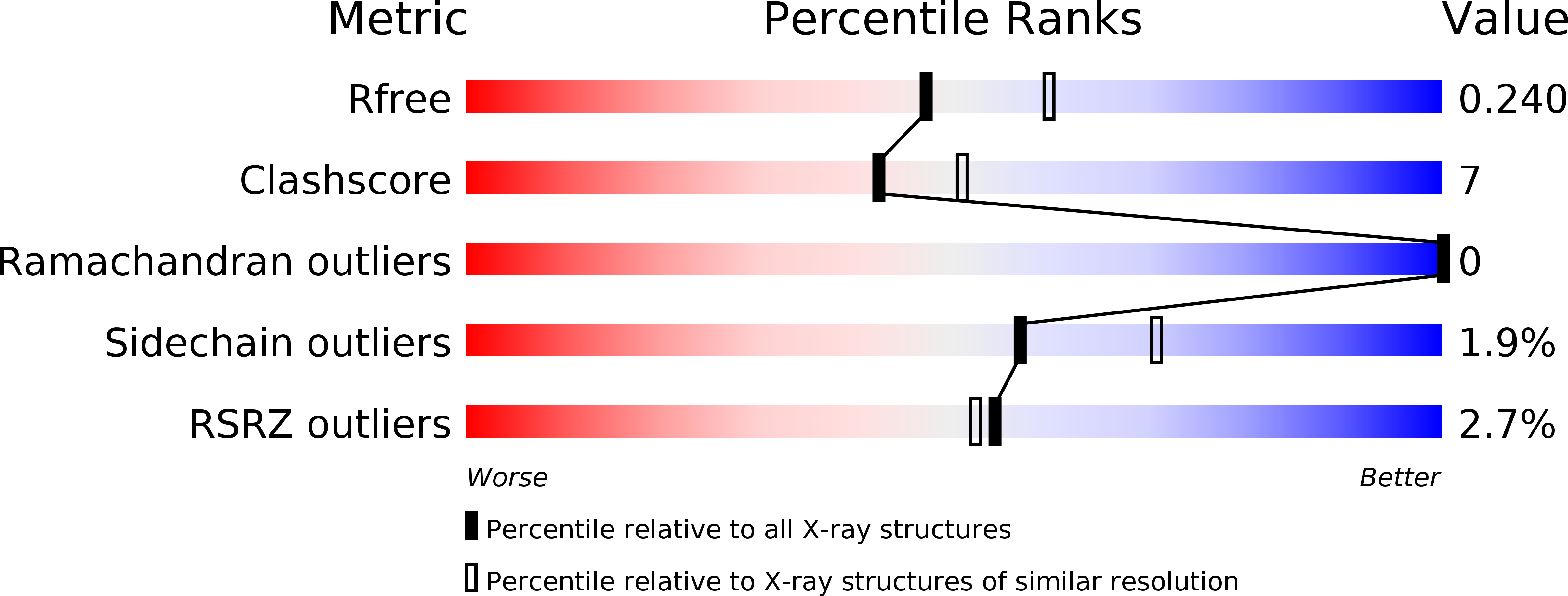
R-Value Free:
0.24
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 21 3