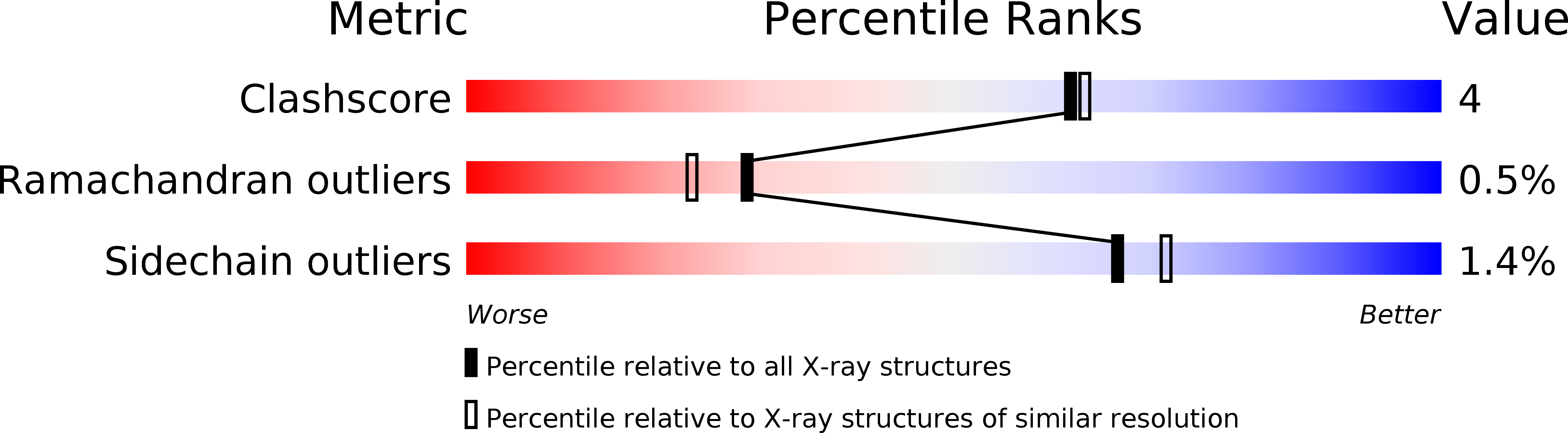Deposition Date
2004-01-10
Release Date
2004-02-19
Last Version Date
2023-12-13
Entry Detail
PDB ID:
1UUT
Keywords:
Title:
The Nuclease Domain of Adeno-Associated Virus Rep Complexed with the RBE' Stemloop of the Viral Inverted Terminal Repeat
Biological Source:
Source Organism(s):
ADENO-ASSOCIATED VIRUS 5 (Taxon ID: 82300)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.00 Å
R-Value Free:
0.23
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 21 21 21