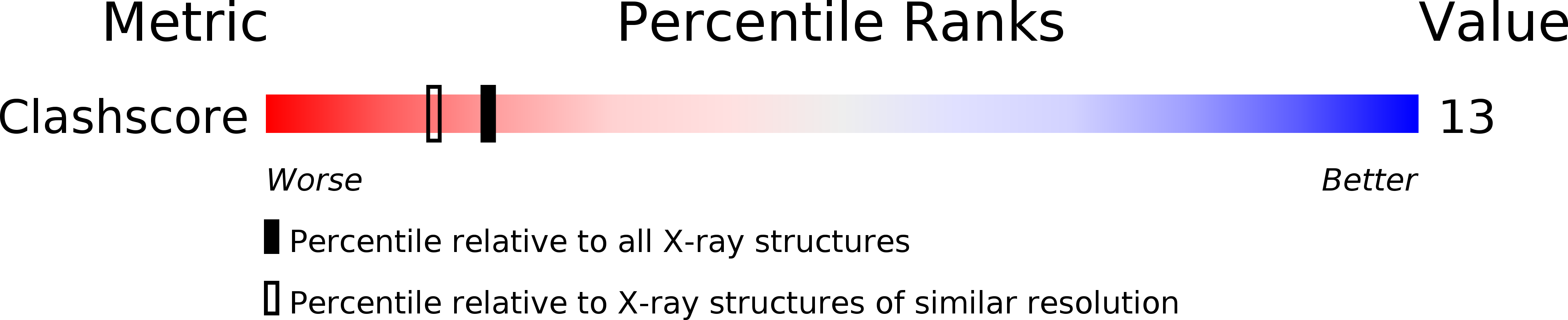Deposition Date
2003-11-28
Release Date
2004-01-02
Last Version Date
2024-11-20
Entry Detail
PDB ID:
1USR
Keywords:
Title:
Newcastle disease virus hemagglutinin-neuraminidase: Evidence for a second sialic acid binding site and implications for fusion
Biological Source:
Source Organism(s):
NEWCASTLE DISEASE VIRUS (Taxon ID: 11176)
Method Details:
Experimental Method:
Resolution:
2.00 Å
R-Value Free:
0.22
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 21 21 2