Deposition Date
2003-11-20
Release Date
2004-09-01
Last Version Date
2023-12-13
Entry Detail
PDB ID:
1USB
Keywords:
Title:
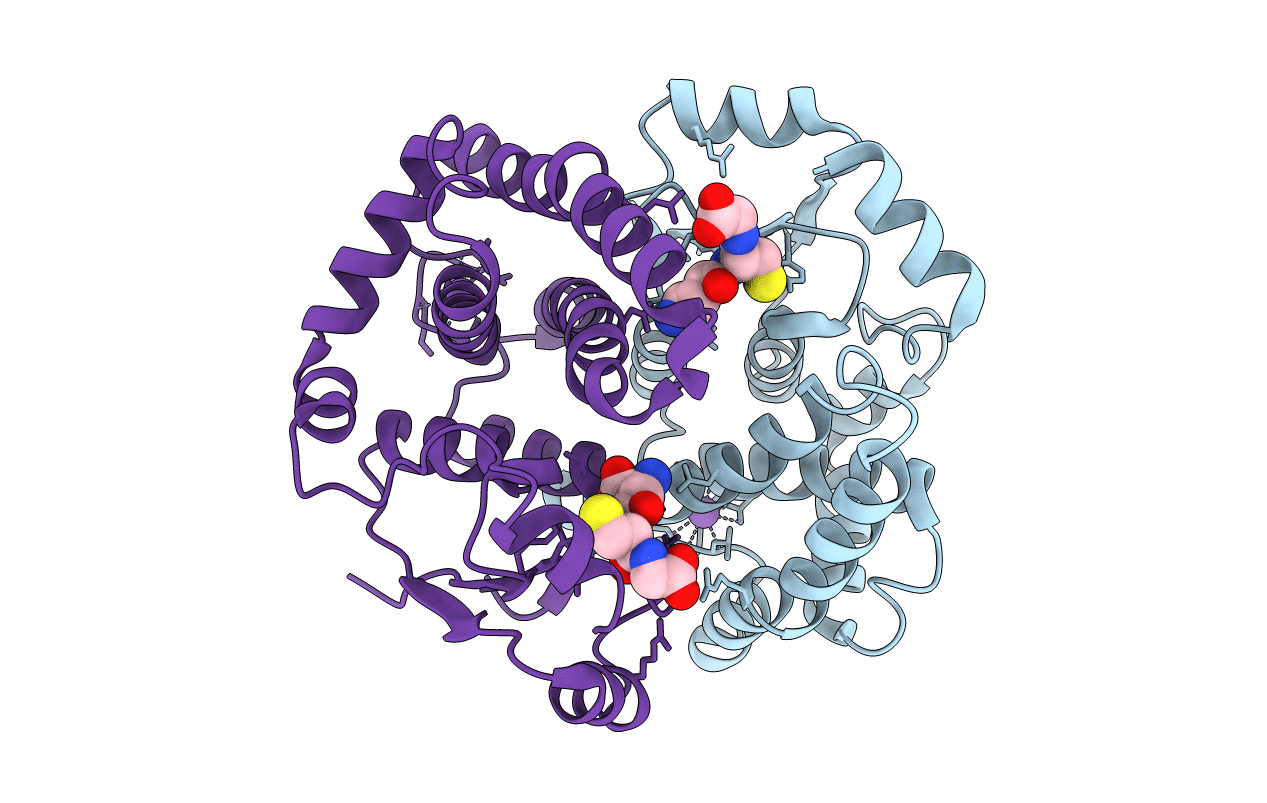
Rational design of a novel enzyme - efficient thioester hydrolysis enabled by the incorporation of a single His residue into human glutathione transferase A1-1
Biological Source:
Source Organism(s):
HOMO SAPIENS (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
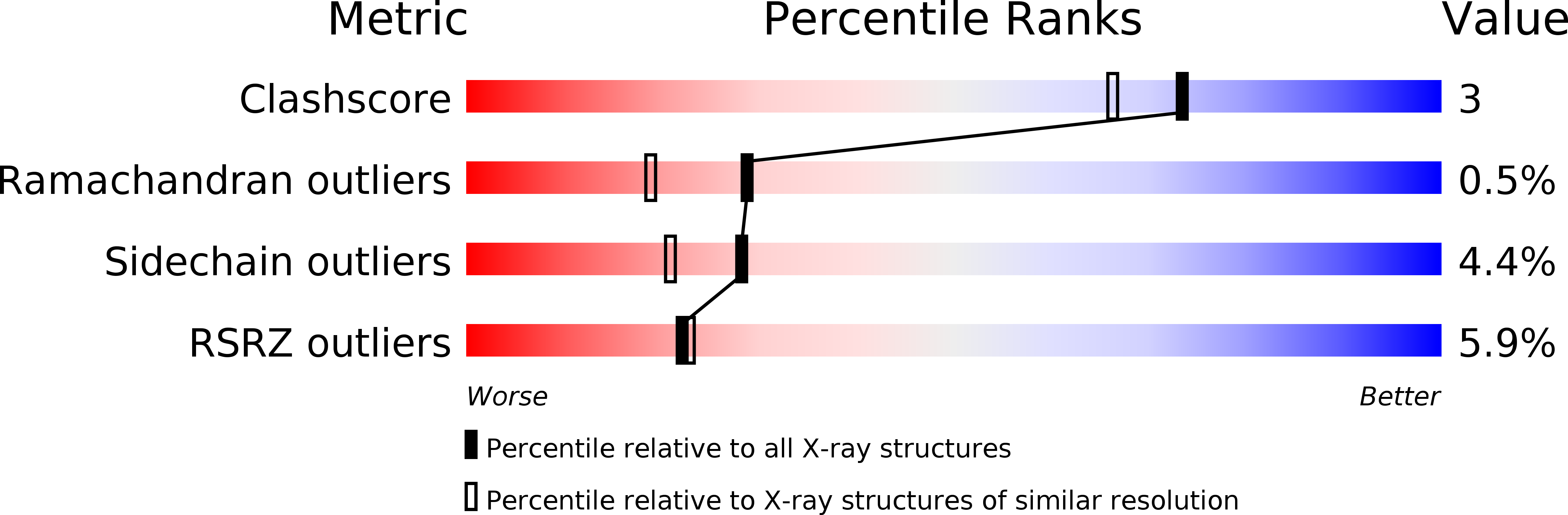
Resolution:
2.07 Å
R-Value Free:
0.24
R-Value Work:
0.18
R-Value Observed:
0.19
Space Group:
C 1 2 1