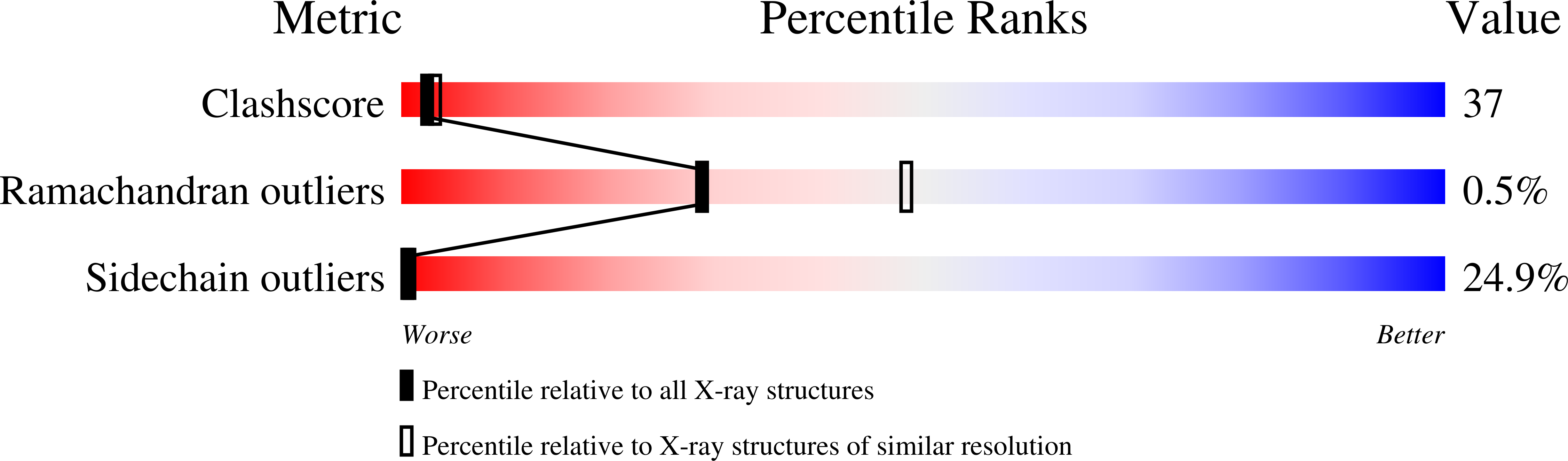Deposition Date
1998-04-16
Release Date
1999-05-11
Last Version Date
2024-02-14
Entry Detail
PDB ID:
1UPU
Keywords:
Title:
STRUCTURE OF THE URACIL PHOSPHORIBOSYLTRANSFERASE, MUTANT C128V, BOUND TO PRODUCT URIDINE-1-MONOPHOSPHATE (UMP)
Biological Source:
Source Organism(s):
Toxoplasma gondii (Taxon ID: 5811)
Expression System(s):
Method Details: