Deposition Date
2003-10-02
Release Date
2004-10-05
Last Version Date
2023-10-25
Entry Detail
PDB ID:
1UMJ
Keywords:
Title:
Crystal structure of Pyrococcus horikoshii CutA in the presence of 3M guanidine hydrochloride
Biological Source:
Source Organism:
Pyrococcus horikoshii (Taxon ID: 70601)
Host Organism:
Method Details:
Experimental Method:
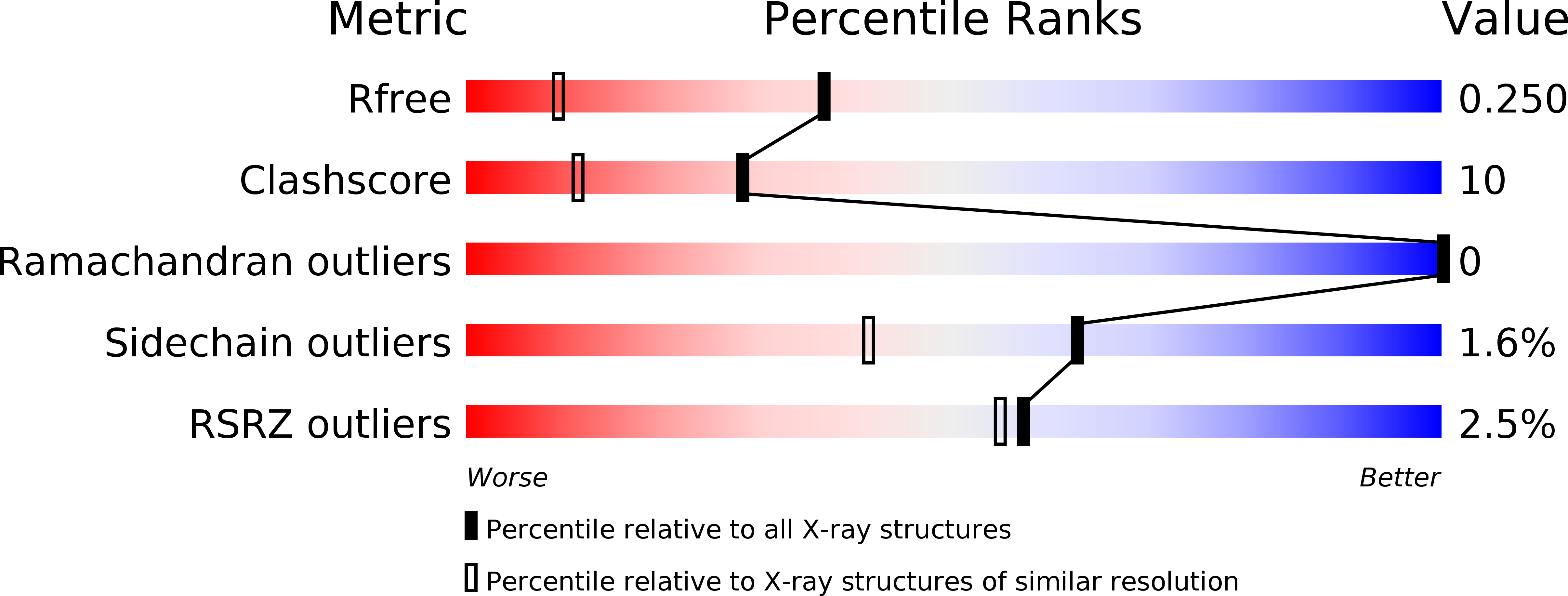
Resolution:
1.60 Å
R-Value Free:
0.25
R-Value Work:
0.21
Space Group:
P 3