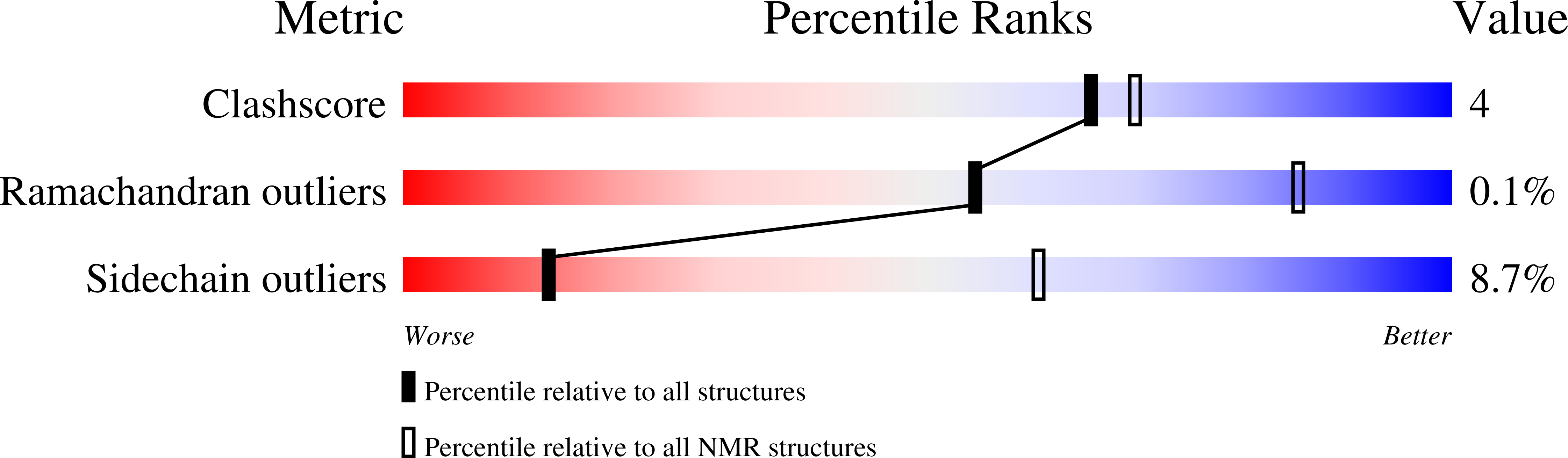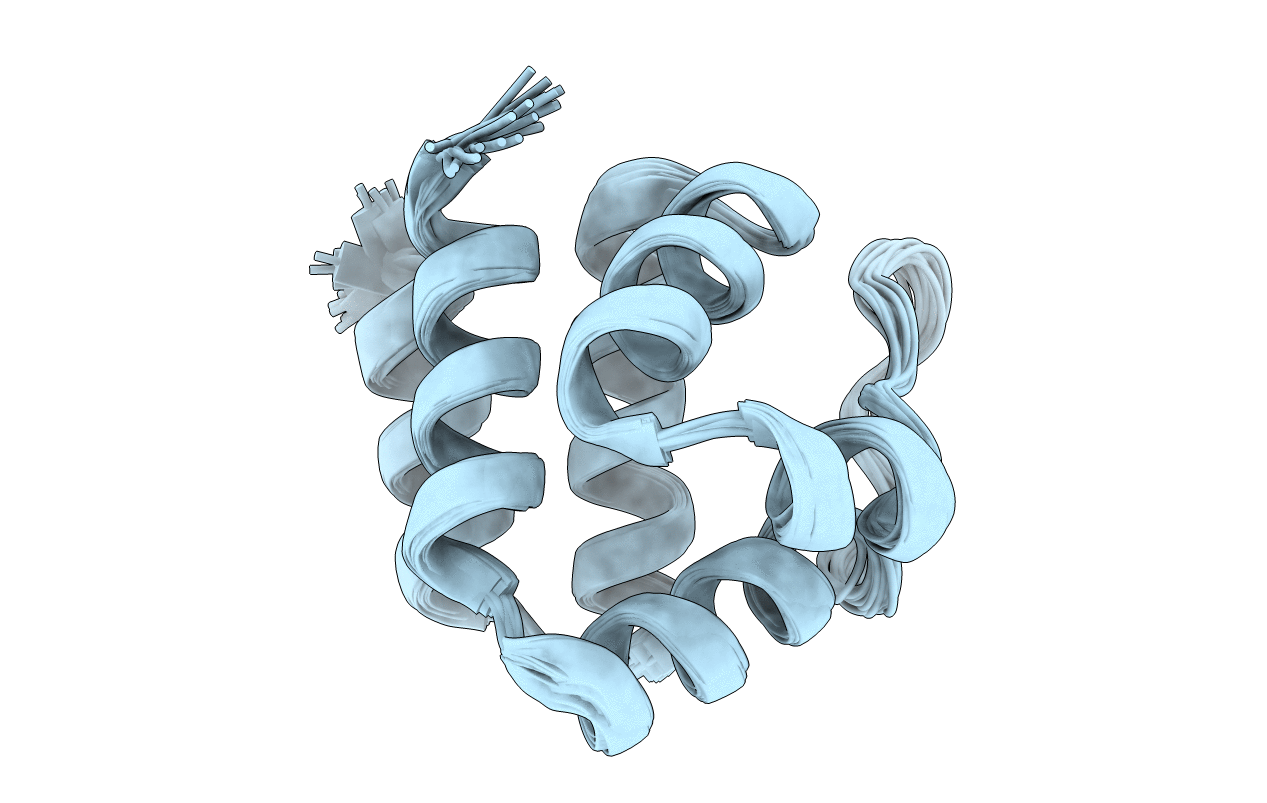
Deposition Date
2003-04-16
Release Date
2003-11-04
Last Version Date
2023-12-27
Entry Detail
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
20
Selection Criteria:
structures with the least restraint violations,target function