Deposition Date
2004-08-09
Release Date
2004-11-23
Last Version Date
2024-02-14
Entry Detail
PDB ID:
1U8Y
Keywords:
Title:
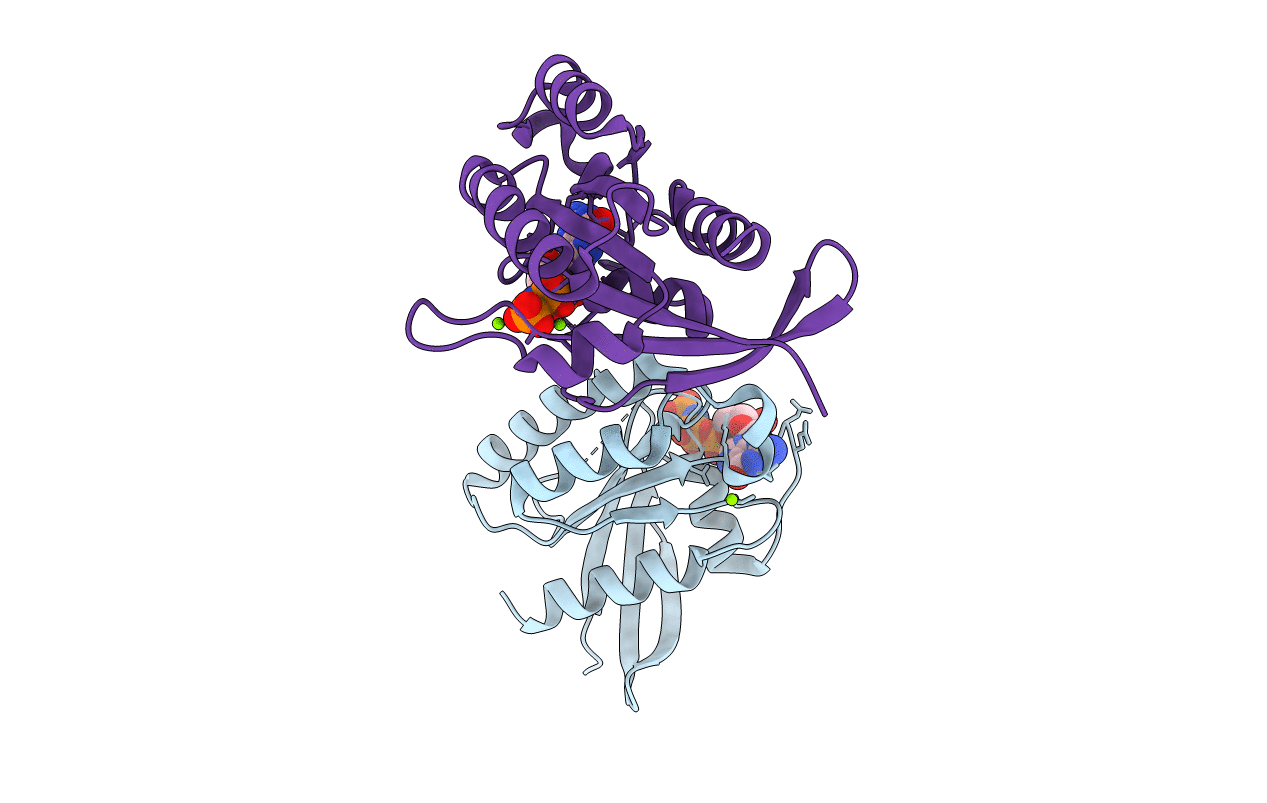
CRystal structures of Ral-GppNHp and Ral-GDP reveal two novel binding sites that are also present in Ras and Rap
Biological Source:
Source Organism(s):
Saguinus oedipus (Taxon ID: 9490)
Expression System(s):
Method Details:
Experimental Method:
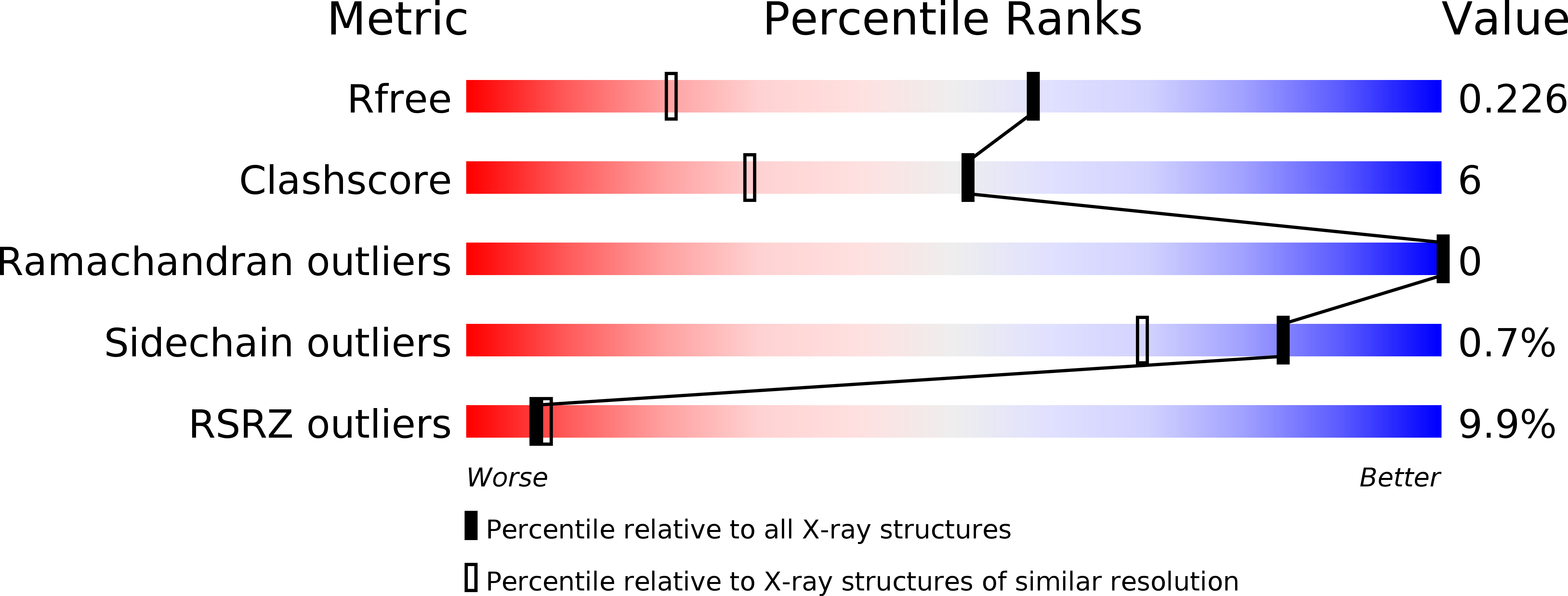
Resolution:
1.55 Å
R-Value Free:
0.23
R-Value Work:
0.21
Space Group:
P 42 2 2