Deposition Date
2004-08-06
Release Date
2004-11-02
Last Version Date
2025-03-26
Entry Detail
PDB ID:
1U8G
Keywords:
Title:
Crystal structure of a HIV-1 Protease in complex with peptidomimetic inhibitor KI2-PHE-GLU-GLU-NH2
Biological Source:
Source Organism:
Human immunodeficiency virus 1 (Taxon ID: 11676)
Host Organism:
Method Details:
Experimental Method:
Resolution:
2.20 Å
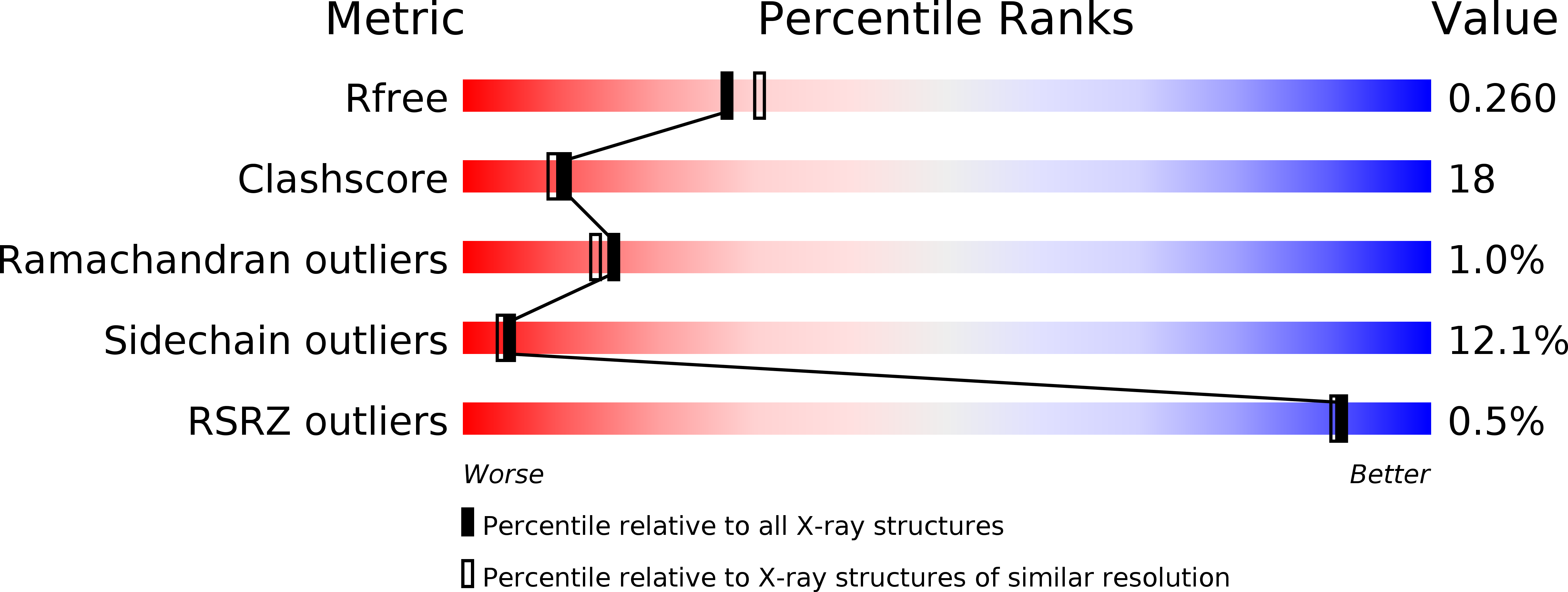
R-Value Free:
0.25
R-Value Work:
0.20
R-Value Observed:
0.21
Space Group:
P 61