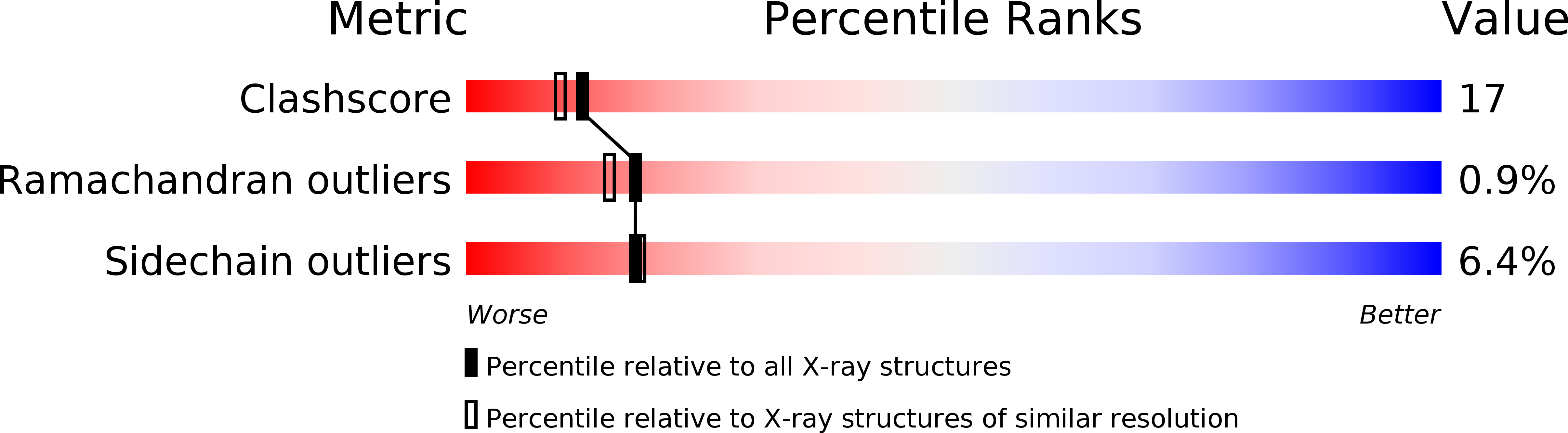Deposition Date
2004-07-26
Release Date
2004-08-10
Last Version Date
2024-11-13
Entry Detail
PDB ID:
1U4J
Keywords:
Title:
Crystal structure of a carbohydrate induced dimer of group I phospholipase A2 from Bungarus caeruleus at 2.1 A resolution
Biological Source:
Source Organism:
Bungarus caeruleus (Taxon ID: 132961)
Method Details:
Experimental Method:
Resolution:
2.18 Å
R-Value Free:
0.22
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
H 3