Deposition Date
2004-07-10
Release Date
2004-08-17
Last Version Date
2024-04-03
Entry Detail
PDB ID:
1TZO
Keywords:
Title:
Crystal Structure of the Anthrax Toxin Protective Antigen Heptameric Prepore
Biological Source:
Source Organism:
Bacillus anthracis str. (Taxon ID: 191218)
Host Organism:
Method Details:
Experimental Method:
Resolution:
3.60 Å
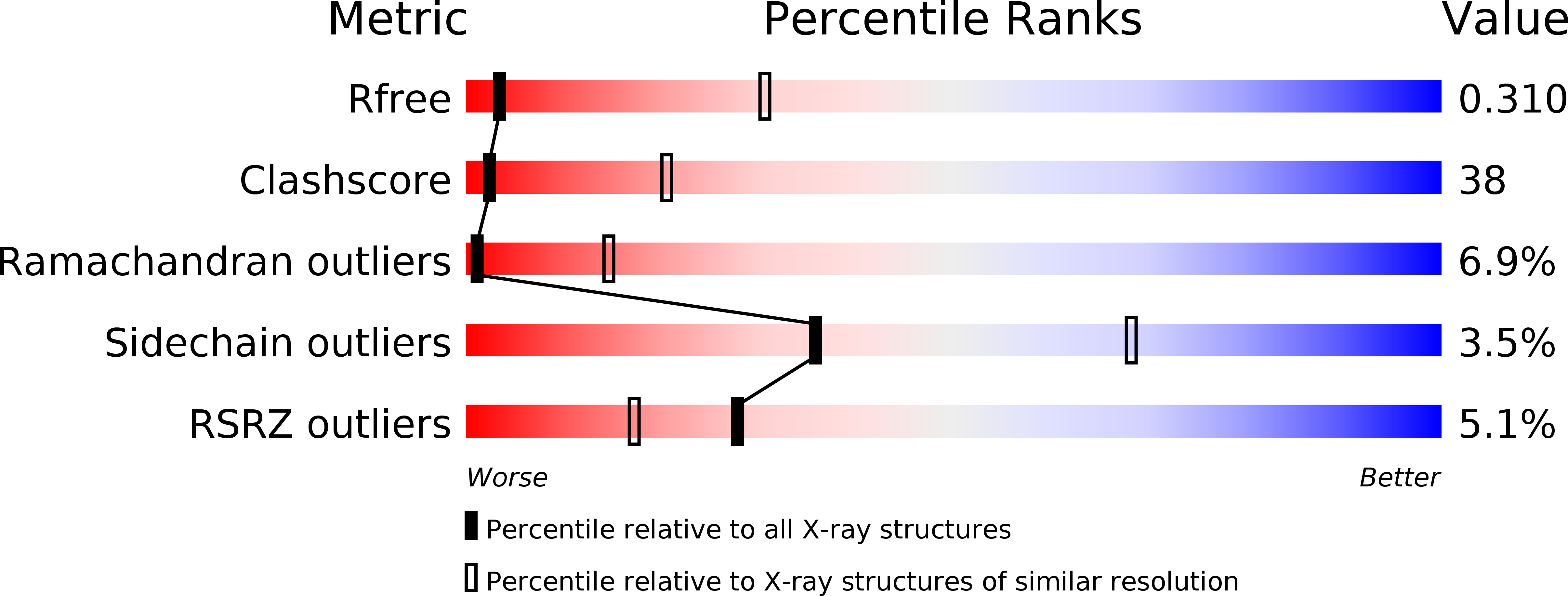
R-Value Free:
0.31
R-Value Work:
0.31
R-Value Observed:
0.31
Space Group:
P 1