Deposition Date
1994-09-14
Release Date
1994-11-30
Last Version Date
2024-10-23
Entry Detail
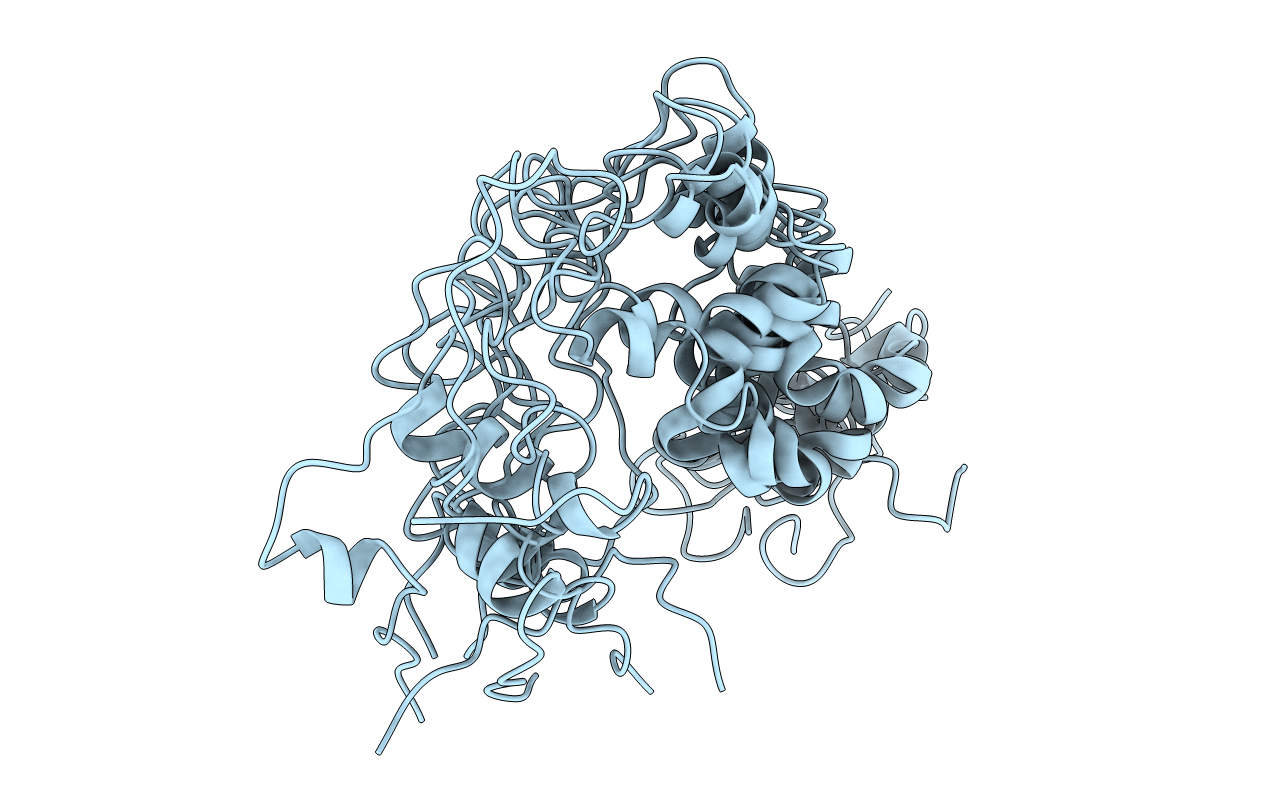
PDB ID:
1TVS
Keywords:
Title:
TRIFLUOROETHANOL STABILIZES A HELIX-TURN-HELIX MOTIF IN EQUINE INFECTIOUS-ANEMIA-VIRUS TRANS-ACTIVATOR PROTEIN
Biological Source:
Source Organism(s):
Equine infectious anemia virus (Taxon ID: 11665)
Method Details:
Experimental Method:
Conformers Submitted:
8


