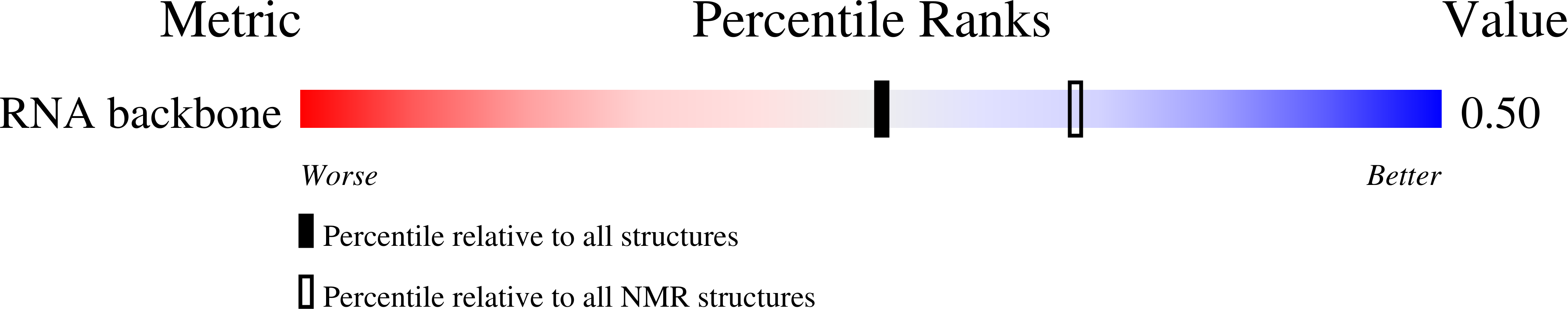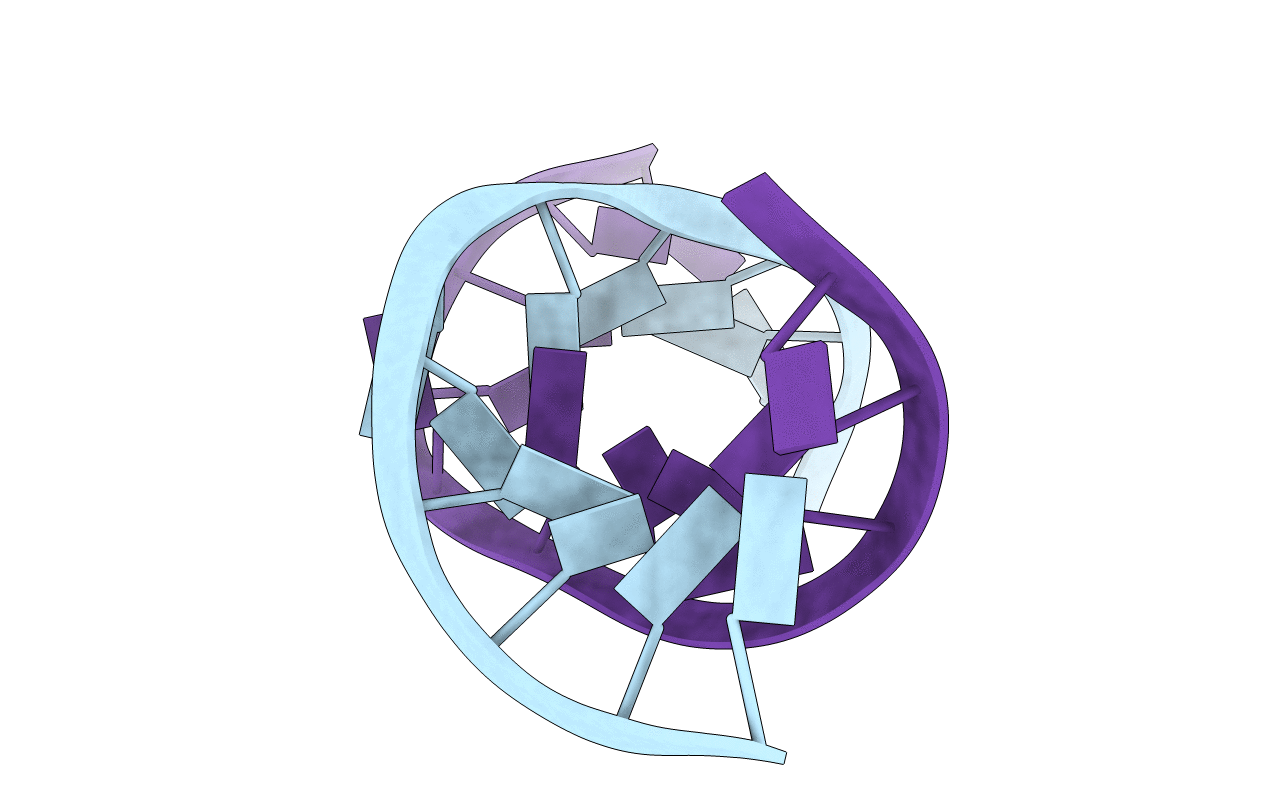
Deposition Date
2004-06-25
Release Date
2004-12-28
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1TUT
Keywords:
Title:
J4/5 Loop from the Candida albicans and Candida dubliniensis Group I Introns
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
1
Selection Criteria:
closest to average