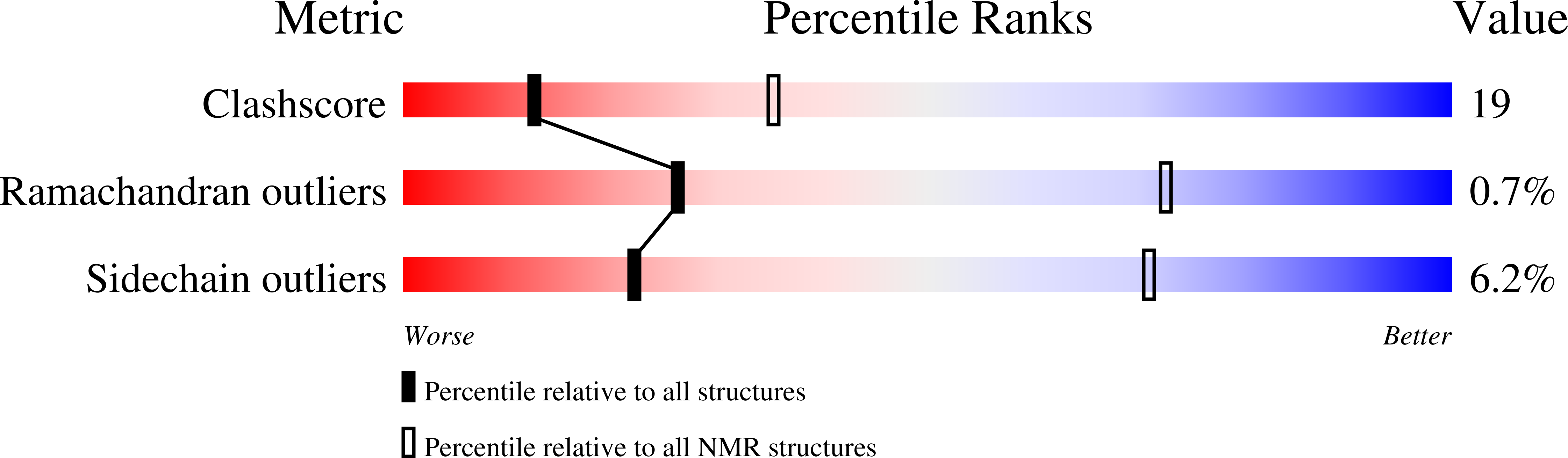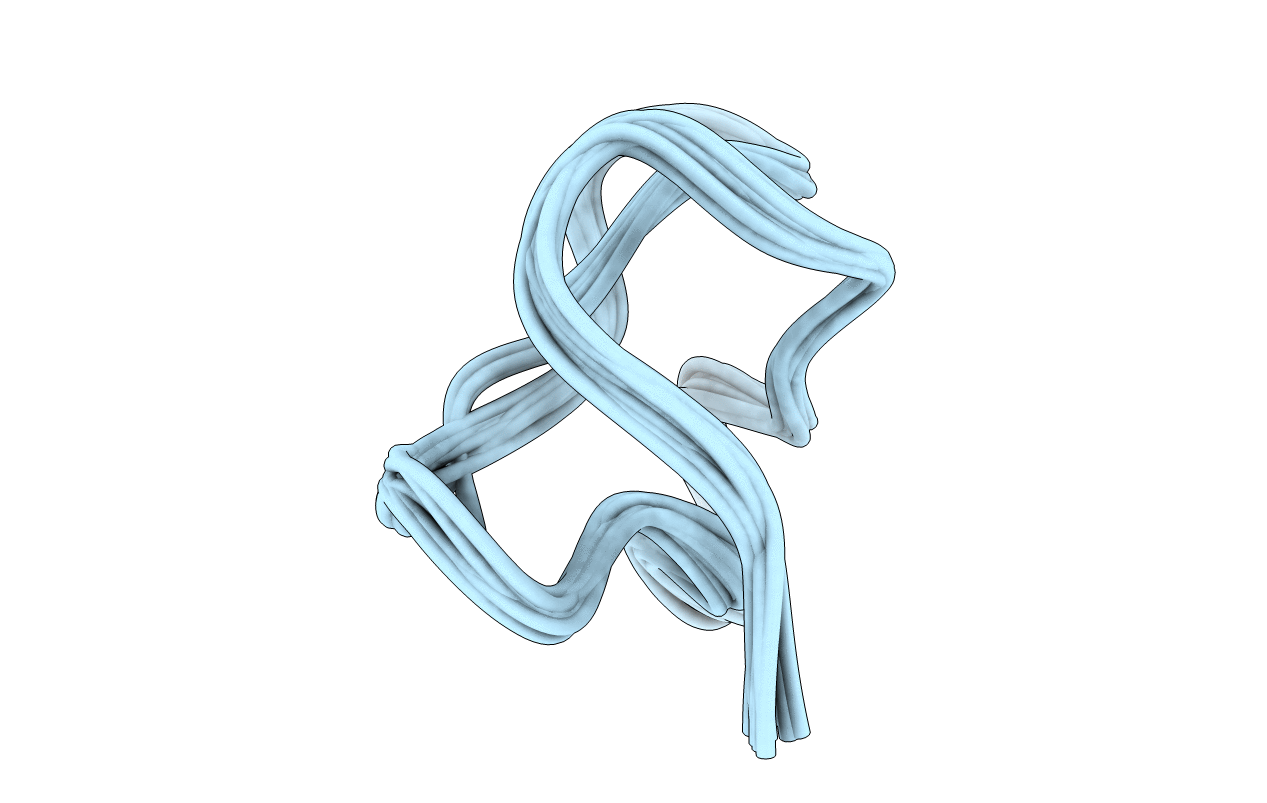
Deposition Date
2004-06-22
Release Date
2004-07-06
Last Version Date
2024-11-06
Entry Detail
PDB ID:
1TTK
Keywords:
Title:
NMR solution structure of omega-conotoxin MVIIA, a N-type calcium channel blocker
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
17
Selection Criteria:
structures with the lowest energy