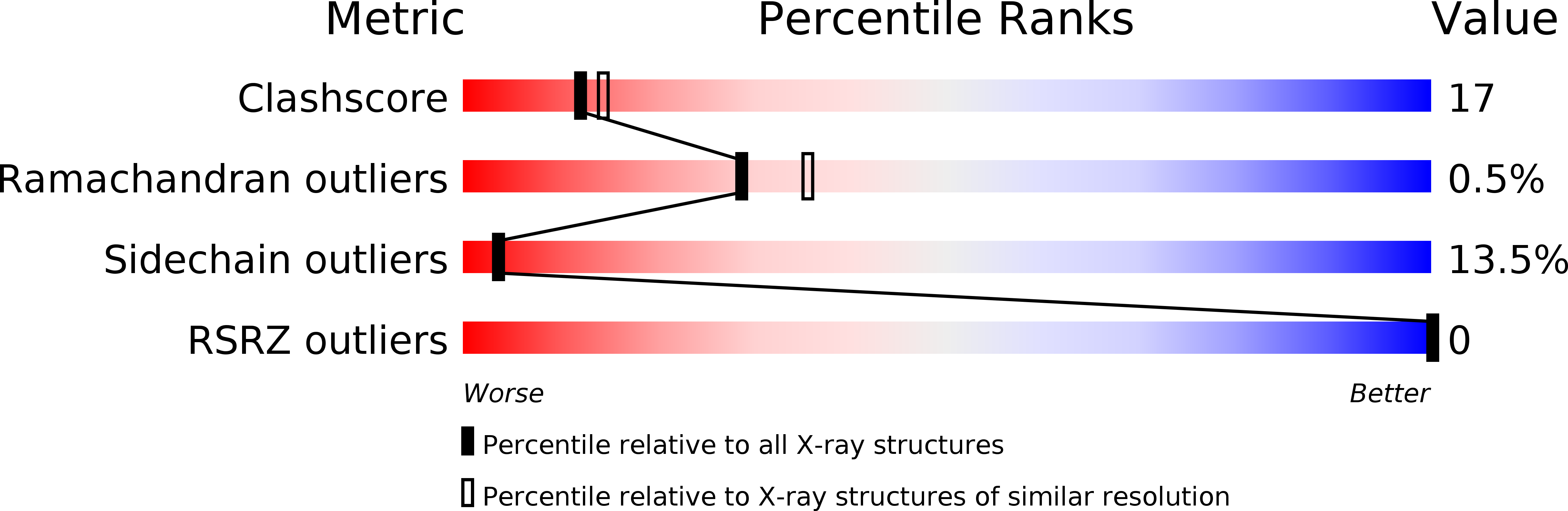Deposition Date
1993-07-06
Release Date
1994-04-30
Last Version Date
2024-10-23
Entry Detail
PDB ID:
1TRQ
Keywords:
Title:
X-RAY CRYSTALLOGRAPHIC AND CALORIMERIC STUDIES OF THE EFFECTS OF THE MUTATION TRP 59 TYR IN RIBONUCLEASE T1
Biological Source:
Source Organism:
Aspergillus oryzae (Taxon ID: 5062)
Method Details:
Experimental Method:
Resolution:
2.30 Å
R-Value Observed:
0.16
Space Group:
P 1 21 1