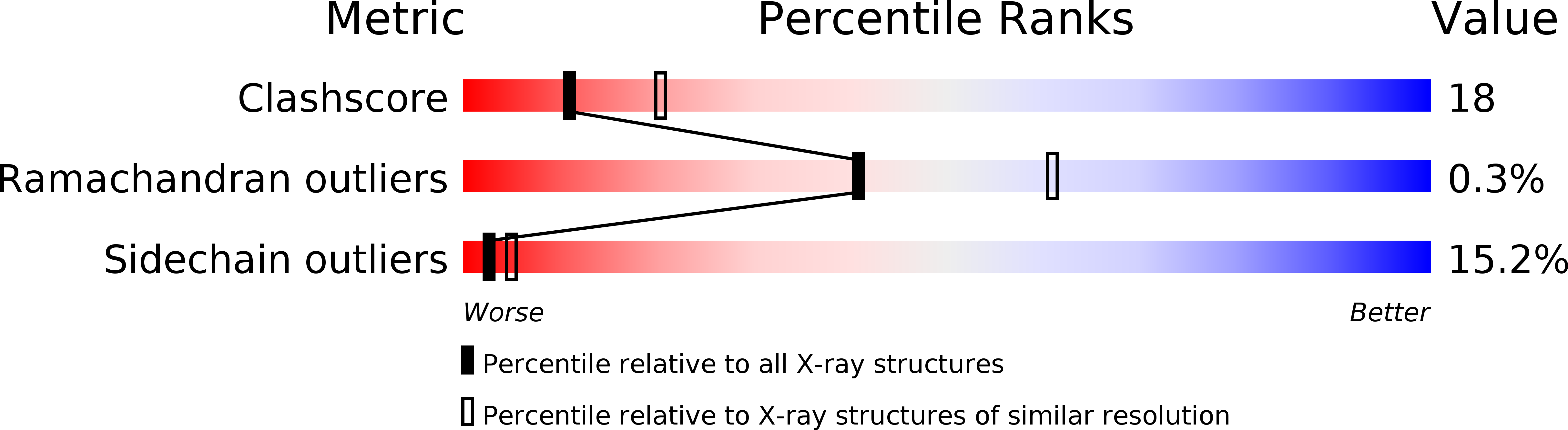Deposition Date
1994-05-26
Release Date
1994-09-30
Last Version Date
2024-10-23
Entry Detail
PDB ID:
1TMU
Keywords:
Title:
Changes in interactions in complexes of hirudin derivatives and human alpha-thrombin due to different crystal forms
Biological Source:
Source Organism(s):
HIRUDO MEDICINALIS (Taxon ID: 6421)
Homo sapiens (Taxon ID: 9606)
Homo sapiens (Taxon ID: 9606)
Method Details:
Experimental Method:
Resolution:
2.50 Å
R-Value Observed:
0.20
Space Group:
P 21 21 2