Deposition Date
2004-06-09
Release Date
2004-12-14
Last Version Date
2023-08-23
Entry Detail
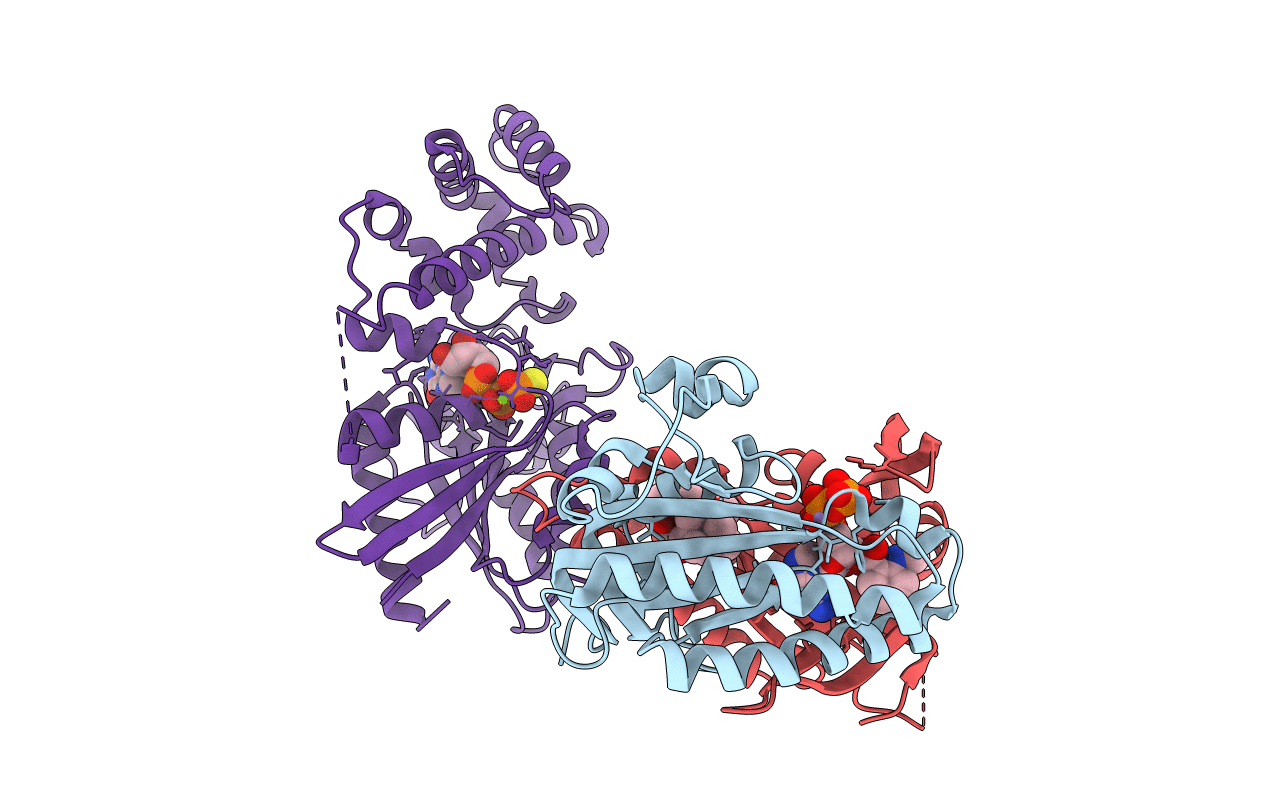
PDB ID:
1TL7
Keywords:
Title:
Complex Of Gs- With The Catalytic Domains Of Mammalian Adenylyl Cyclase: Complex With 2'(3')-O-(N-methylanthraniloyl)-guanosine 5'-triphosphate and Mn
Biological Source:
Source Organism(s):
Canis lupus familiaris (Taxon ID: 9615)
Rattus norvegicus (Taxon ID: 10116)
Bos taurus (Taxon ID: 9913)
Rattus norvegicus (Taxon ID: 10116)
Bos taurus (Taxon ID: 9913)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.80 Å
R-Value Free:
0.29
R-Value Work:
0.25
R-Value Observed:
0.25
Space Group:
P 21 21 2


