Deposition Date
2004-05-21
Release Date
2004-06-08
Last Version Date
2024-10-23
Entry Detail
PDB ID:
1TD7
Keywords:
Title:
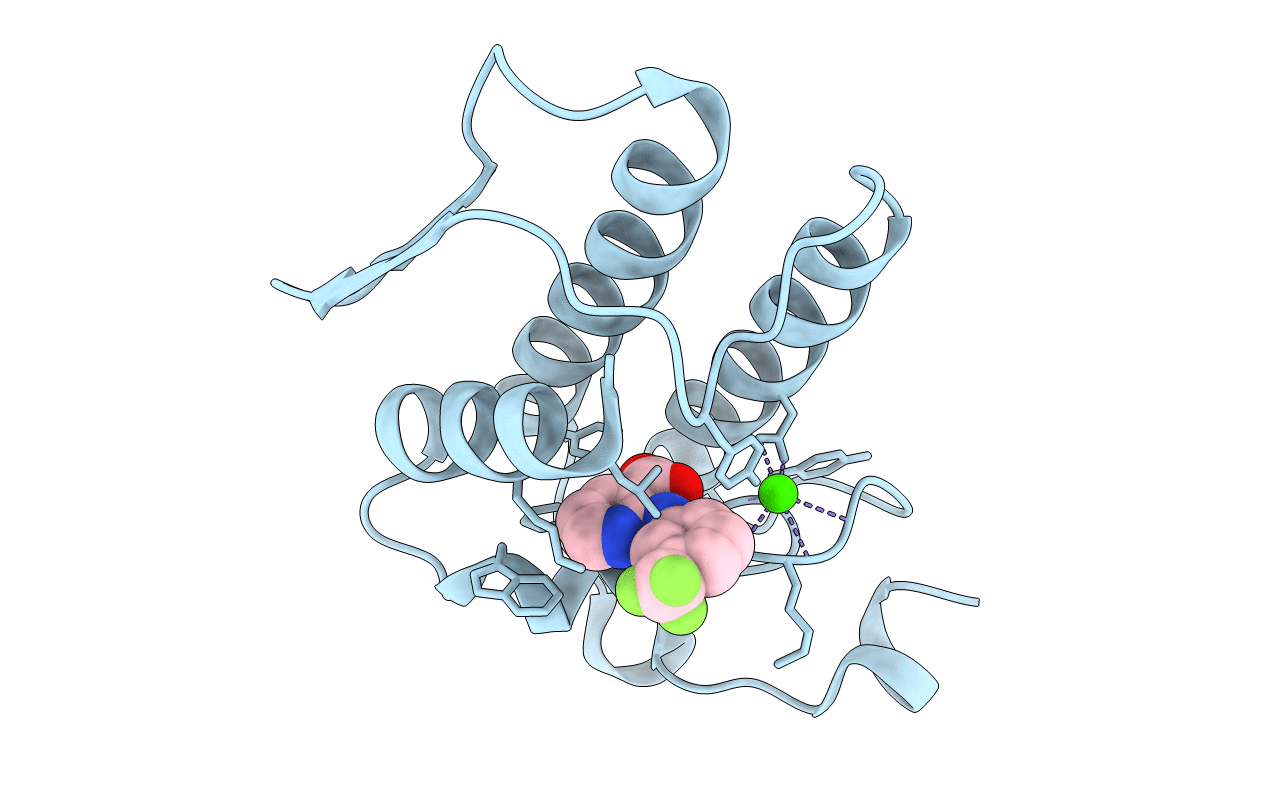
Interactions of a specific non-steroidal anti-inflammatory drug (NSAID) with group I phospholipase A2 (PLA2): Crystal structure of the complex formed between PLA2 and niflumic acid at 2.5 A resolution
Biological Source:
Source Organism(s):
Naja sagittifera (Taxon ID: 195058)
Method Details:
Experimental Method:
Resolution:
2.50 Å
R-Value Free:
0.24
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 41


